《固体物理学》课程教学课件(PPT讲稿)Chapter 7 The electronic theory of metal 7.1 Fermi statistics and the heat capacity of electron 7.2 Work function and contact potential

Chapter 7 The electronic theory of metal Objectives At the end of this Chapter,you should: 1.Understand the physical meaning of Fermi statistical distribution function. 2.Be able to calculate the Fermi level at 0 K. 3.Understand the concept of work function and contact potential. 4.Be able to calculate the heat capacity of electrons for metals
Chapter 7 The electronic theory of metal Objectives At the end of this Chapter, you should: 1. Understand the physical meaning of Fermi statistical distribution function. 2. Be able to calculate the Fermi level at 0 K. 3. Understand the concept of work function and contact potential. 4. Be able to calculate the heat capacity of electrons for metals

7.1 Fermi statistics and the heat capacity of electron The function of Fermi distribution For the equilibrium state of one system,the basic principle of Fermi statistics can be 1 f(E)=eF-+1 Which the probability that the eigenstate at energy E will be occupied by an electron
7.1 Fermi statistics and the heat capacity of electron For the equilibrium state of one system, the basic principle of Fermi statistics can be k 1 ( ) 1 E E T F B f E e − = + Which the probability that the eigenstate at energy E will be occupied by an electron. The function of Fermi distribution

The function of Fermi distribution f(E)has the form 1)E=Ee,f(E)=1/2 E 2)When E is higher than Er by several kBT, T=0K e5-Ee/kr>1,f(E)≈0 such eigenstate is almost empty; 3)When E is lower than Er by several kpT, 0.5 1f(E) eB-Erkr<l,f(E)≈1 The function of shows that the function f(E)changes from Fermi distribution value 1 to value 0 within the scope of several kBT up and down Er
The function of Fermi distribution f (E) has the form , ( ) 1 2 E E f E = = F 1, ( ) 0 E E k T F B e f E − k 1, ( ) 1 E E T F B e f E − The function of shows that the function f (E) changes from Fermi distribution value 1 to value 0 within the scope of several kBT up and down EF . such eigenstate is almost empty; 3) When E is lower than EF by several kBT, 2)When E is higher than EF by several kBT, 1)
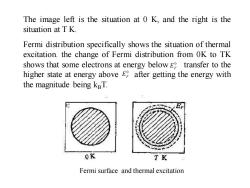
The image left is the situation at 0 K,and the right is the situation at TK. Fermi distribution specifically shows the situation of thermal excitation.the change of Fermi distribution from OK to TK shows that some electrons at energy below E transfer to the higher state at energy above E after getting the energy with the magnitude being kBT. 0K TK Fermi surface and thermal excitation
The image left is the situation at 0 K, and the right is the situation at T K. Fermi distribution specifically shows the situation of thermal excitation. the change of Fermi distribution from 0K to TK shows that some electrons at energy below transfer to the higher state at energy above after getting the energy with the magnitude being kBT. Fermi surface and thermal excitation 0 EF 0 EF 0 EF

Density of States N(E) We often need to know the density of electron states,which is the number of states per unit energy,so we can quickly calculate it: The differential number of electron states in a range of energy dE or wavevector dk is: dN N(EdE=N(k)dk N(E)=N(k d_N(k)_V/8π3mV dEdE/dk2k/m28π3k Now using the general relation: 1/2 k=司 2mE we get: C2mE)
Density of States N(E) We often need to know the density of electron states, which is the number of states per unit energy, so we can quickly calculate it: dN = N(E)dE = N(k)dk k mV k m V dE dk N k dE dk N E N k 2 2 3 3 / 8 /8 / ( ) ( ) ( ) = = = = Now using the general relation: 1/ 2 2 2 = mE k we get: ( ) 1/ 2 3 2 3 ( ) 2m E V N E = The differential number of electron states in a range of energy dE or wavevector dk is:
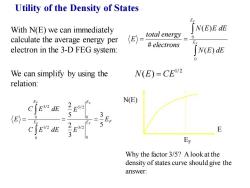
Utility of the Density of States With N(E)we can immediately 「N(E)EdE calculate the average energy per (E〉= total energy 0 electron in the 3-D FEG system: electrons N(E)dE We can simplify by using the N(E)=CE12 relation: 2 EF N(E) C∫EndE E5/2 5 (E〉= 0 0 3 E 2 C E2 dE E32 3 Er Why the factor 3/5?Alook at the density of states curve should give the answer:
Utility of the Density of States We can simplify by using the relation: With N(E) we can immediately calculate the average energy per electron in the 3-D FEG system: Why the factor 3/5? A look at the density of states curve should give the answer: = = F F E E N E dE N E E dE electrons total energy E 0 0 ( ) ( ) # 1/ 2 N(E) = CE E F E E E E E E C E dE C E dE E F F F F 5 3 3 2 5 2 0 3/ 2 0 5/ 2 0 1/ 2 0 3/ 2 = = = N(E) E EF

we often need to know the distribution of the electronic energy, then we can get the statistical average number of electrons according to the Fermi distribution function is: f(E)N(E)dE N(E) (E)N(E)rg Cf(E)N(E)JoR The density of energy state and the electron distribution in accordance with energy
The density of energy state and the electron distribution in accordance with energyf E dE ( )N E( ) we often need to know the distribution of the electronic energy, then we can get the statistical average number of electrons according to the Fermi distribution function is :

Determination of Er In the system,the total number of electron is given by N=f(E)N(E)dE Introducing a new function (E)=fN(E)dE Q(E)means the total number of the quantum states with energy above E. Then we can obtain N-E5)) Because Q(E)=0 when E-0,and f(E)-0 when E=oo,The first is zero.So af dE
Determination of EF ( ) 0 N f E dE ( )N E = ( ) 0 ( ) N E E Q E dE = 0 ( ) ( ) ( ) 0 N f E Q E Q E dE f E + = − In the system , the total number of electron is given by 0 N Q E( ) E E f d = − Introducing a new function Because Q(E) =0 when E=0, and f (E)=0 when E=∞, The first is zero. So Q (E) means the total number of the quantum states with energy above E. Then we can obtain
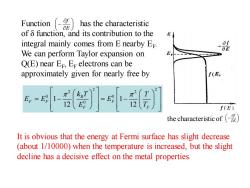
Fuction has the characteristic of 8 function,and its contribution to the integral mainly comes from E nearby Ee. of OE We can perform Taylor expansion on E Q(E)near E,Er electrons can be approximately given for nearly free by f(E Er E 12 f(E) the characteristic of It is obvious that the energy at Fermi surface has slight decrease (about 1/10000)when the temperature is increased,but the slight decline has a decisive effect on the metal properties
Function has the characteristic of δ function, and its contribution to the integral mainly comes from E nearby EF . We can perform Taylor expansion on Q(E) near EF , EF electrons can be approximately given for nearly free by f E − 2 2 2 2 0 0 0 1 1 12 12 B F F F F F k T T E E E E T = − = − It is obvious that the energy at Fermi surface has slight decrease (about 1/10000) when the temperature is increased, but the slight decline has a decisive effect on the metal properties. the characteristic of f E −

The heat capacity of electron 19th century puzzle:each monatomic gas molecule in sample at temperature T3kT has energy E=N(k),so if the N free electrons in a metal make up a classical "gas"they should behave similarly. 月-7-N,k灯-m n n So the electronic contribution to the molar heat capacity would be expected to be d(Ea Ca-arn =多R This is half of the 3R we found for the lattice heat capacity at high T.But experiments show that the total C for metals is only slightly higher than for insulatorswhich conflicts with the classical theory!
So the electronic contribution to the molar heat capacity would be expected to be k T N k T RT n N n E A el 2 3 2 3 2 3 = = = R n E dT d C el el 2 3 = = This is half of the 3R we found for the lattice heat capacity at high T. But experiments show that the total C for metals is only slightly higher than for insulators—which conflicts with the classical theory! ( ) 2 3 Eel = N kT 19th century puzzle: each monatomic gas molecule in sample at temperature T has energy , so if the N free electrons in a metal make up a classical “gas” they should behave similarly. kT 2 3 The heat capacity of electron
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 7 The electronic theory of metal(supplement - Sommerfield expansion).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 6 Electron motion in the crystal 6.4 The electron motion in a constant electric field 6.5 Band structure of conductor, insulator and semiconductor.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 6 Electron motion in the crystal 6(supplement - a more concise description of quasi-momentum).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 6 Electron motion in the crystal 6.1 The quasi-classical description of Bloch electrons 6.2. Electron quasi-momentum 6.3 The accelerated velocity and effective mass.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 5 band theory 5.4 Tight Binding Approximation(TBA).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 5 band theory 5.3 Nearly Free Electron 5.4 BZ and energy bands.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 5 band theory 5.5 The symmetry of bands.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 4 lattice dynamics and lattice capacity 4(supplement - Thermal Expansion of Glass).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 4 lattice dynamics and lattice capacity 4.6 Anharmonic Effects 4.7 Equation of states for Lattice 4.8 Experimental methods for the determinations of lattice vibration spectroscopy.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 5 band theory 5.1 general background and approximations 5.2 Bloch’s Theorem.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 4 lattice dynamics and lattice capacity 4.5 Heat capacity of the lattice.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 4 lattice dynamics and lattice capacity 4.1 Normal Modes of Vibration 4.2 Density of States 4.3 Harmonic approximation and normal mode coordinates 4.4 phonon.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 3 Interatomic Bonding 3.1 General crystal binding 3.2 typical binding.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 3 Interatomic Bonding 3(Supplement - Rules of crystal binding).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 3 Interatomic Bonding 3.3 The cohesive energy for ionic crystal.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 2 Wave Diffraction & the Reciprocal Lattice(2.1-2.5).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 2 Wave Diffraction & the Reciprocal Lattice 2.6 X-Ray Diffraction Methods 2.7 Applications of XRD.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 2 Wave Diffraction & the Reciprocal Lattice(supplement - Review & Overview of X-Rays).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 1 Crystal Structure 1.9 Elements of Symmetry 1.10 Space groups 1.11 7 crystal system and 14 Bravis Lattice.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 1 Crystal Structure(Supplement - microstructure and crystal system).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 5 band theory 5.6 Density of states(DOS)and Fermi surface 5.7 the electrons in the crystal 5.8 the experimental results for DOS.ppt
- 《近代物理实验》课程教学大纲.doc
- 《近代物理实验》课程教学资源(PPT课件讲稿,共五部分).ppt
- 《工程光学》课程教学大纲 Engineering Optics.doc
- 安徽大学:《工程光学》课程授课教案(讲义,共十三章,授课教师:李桂华).doc
- 《工程光学》课程实验指导书(共六个实验).doc
- 《量子力学》课程教学课件(讲稿)Chapter 4 态和力学量的表象 The representation for the states and dynamical variable.pdf
- 《量子力学》课程教学课件(讲稿)Chapter 1 绪论 Quantum mechanism(量子力学的诞生).pdf
- 《量子力学》课程教学课件(讲稿)Chapter 2 波函数和薛定谔方程 The wave function and Schrödinger Equation.pdf
- 《量子力学》课程教学课件(讲稿)Chapter 3 量子力学中的力学量 The Dynamical variable in Quantum Mechanism.pdf
- 《量子力学》课程教学课件(讲稿)Chapter 6 散射 Scattering.pdf
- 《量子力学》课程教学课件(讲稿)Chapter 5 微扰理论近似方法 Approximation(本征值问题的似解).pdf
- 《量子力学》课程教学课件(讲稿)Chapter 7 自旋与角动量、全同粒子体系 Spin and undistinguished similar particles.pdf
- 《量子力学》课程教学资源(习题解答)第七章 自旋与角动量、全同粒子体系.doc
- 《量子力学》课程教学资源(习题解答)第三章 量子力学中的力学量.doc
- 《量子力学》课程教学资源(习题解答)第二章 波函数和薛定谔方程.doc
- 《量子力学》课程教学资源(习题解答)第五章 微扰理论.doc
- 《量子力学》课程教学资源(习题解答)第四章 态和力学量的表象.doc
- 《量子力学》课程考试大纲(自考).doc
- 《量子力学》课程教学资源(习题解答)第一章 量子理论基础.doc
