《固体物理学》课程教学课件(PPT讲稿)Chapter 5 band theory 5.1 general background and approximations 5.2 Bloch’s Theorem

Chapter 5 band theory Objectives At the end of this Chapter,you should: 1.Be able to understand the Bloch's Theorem. 2.Know how to calculate the 1D band gap using NFE model 3.Be able to calculate the band gap and band width of a crystal using the TBA model. 4.Have established the concept of band symmetry based on the crystal structure symmetry. 5.Know how to calculate the density of states 6.Understand the concept of Fermi surface
Objectives At the end of this Chapter, you should: 1.Be able to understand the Bloch’s Theorem. 2. Know how to calculate the 1D band gap using NFE model. 3. Be able to calculate the band gap and band width of a crystal using the TBA model. 4. Have established the concept of band symmetry based on the crystal structure symmetry. 5. Know how to calculate the density of states. 6. Understand the concept of Fermi surface. Chapter 5 band theory

5.1 general background and approximations Band theory is to study the solid state electronics,and is the most important theoretical basis for solids,which is the most direct and important results of the quantum mechanics and quantum statistics when applied to the solid.Band theory not only succeeds in solving many problems which is unsolved by the classical electron theory and Sommerfeld free-electron theory,and explain all the crystal properties History: 1)First proposed in 1928(23y),in his doctoral dissertation"on the quantum mechanics in lattice",Bloch first put forward the concept. 2)In 1931,Wilson illustrates the difference between insulators and metal using the band theory and lay the foundation of theory of semiconductor physics. 3)improve the band. The band theory is stricter than the free electrons theory,but it is still an approximate theory
Band theory is to study the solid state electronics, and is the most important theoretical basis for solids, which is the most direct and important results of the quantum mechanics and quantum statistics when applied to the solid. Band theory not only succeeds in solving many problems which is unsolved by the classical electron theory and Sommerfeld free-electron theory, and explain all the crystal properties History: 1) First proposed in 1928(23y), in his doctoral dissertation "on the quantum mechanics in lattice " , Bloch first put forward the concept. 2) In 1931, Wilson illustrates the difference between insulators and metal using the band theory and lay the foundation of theory of semiconductor physics. 3) improve the band . The band theory is stricter than the free electrons theory, but it is still an approximate theory 5.1 general background and approximations

Electrons in Periodic Crystals:Reductionto a One Electron Problem The Schrodinger Equation Hy=Ey(time independent)! Hamiltonian for a Perfect,Periodic Crystal Ne electrons,Nn nuclei;Ne,N~1023 (huge!)Notation:i=electron;j=nucleus 月-+Σ e2 台2m 248,后-3 1 Ze2 4R-R台4心 Approximation#1:Separate the electrons into core Electrons valence Electrons Core Electrons:Those in filled,inner shells of the atoms.Play NO role in determining the electronic properties of the solid! The Si free atom electronic configuration:1s22s22p63s23p2 Core Electrons=1s22s22p6 (filled shells!)Localized around the nuclei.play no role in the bonding.=Lump the core shells together with the Nuclei=Ions
Electrons in Periodic Crystals: Reduction to a One Electron Problem The SchrödingerEquation Hψ = Eψ (time independent)! Hamiltonian for a Perfect, Periodic Crystal Ne electrons, Nn nuclei; Ne , Nn ~ 1023 (huge!) Notation:i = electron; j = nucleus Approximation #1: Separate the electrons into core Electrons & valence Electrons • Core Electrons: Those in filled, inner shells of the atoms. Play NO role in determining the electronic properties of the solid! • The Si free atom electronic configuration: 1s22s22p63s23p2 • Core Electrons= 1s22s22p6 (filled shells!) Localized around the nuclei. play no role in the bonding. Lump the core shells together with the Nuclei Ions

Valence Electrons in the unfilled,outer shells of the free atoms.Si free atom electron configuration:1s22s22p63s23p2 Valence Electrons 3s23p2 (unfilled shell!) In the solid,these hybridize with electrons on neighbor atoms. Approximation #2 The Born-Oppenheimer (Adiabatic)Approximation Separates the electron ion motions. A Qualitative (semiquantitative)Justification:the ratio of the electron ion masses is of the order:(m/Mi)~10-3 (<<1)=Classically,the massive ions move much slower than the very small mass electrons! Typical ionic vibrational frequencies:v:~1013 sThe time scale of the ion motion is:t10-13 s.Electronic motion occurs at energies of about a bandgap:Eg=hve =ho~1ev ve~1015 s1=te~10-15s.So,The electrons respond to the ion motion ~instantaneously! In the electron Hamiltonian,He the ions can be treated as stationary!
Valence Electrons in the unfilled, outer shells of the free atoms. Si free atom electron configuration: 1s22s22p63s23p2 Valence Electrons = 3s23p2 (unfilled shell!) • In the solid, these hybridize with electrons on neighbor atoms. Approximation #2 The Born-Oppenheimer (Adiabatic) Approximation Separates the electron & ion motions. A Qualitative (semiquantitative) Justification: the ratio of the electron & ion masses is of the order: (me /Mi ) ~ 10-3 ( << 1) Classically, the massive ions move much slower than the very small mass electrons! Typical ionic vibrational frequencies: υi ~ 1013 s -1 The time scale of the ion motion is: ti ~ 10-13 s .Electronic motion occurs at energies of about a bandgap:Eg= hυe = ħω ~ 1 eV υe ~ 1015 s -1 te ~ 10-15 s . So, The electrons respond to the ion motion ~ instantaneously! In the electron Hamiltonian, He , the ions can be treated as stationary!

In the ion Hamiltonian,Hi,the electrons can be treated in an average way! The Born-Oppenheimer(Adiabatic)Approximation Write the vibrating ion coordinatesas Ri=Rio+Ri Rjo=equilibrium ion positions,5R=deviation from the equilibrium positions The many body electron-ion Hamiltonian is(schematic!) He-i=He-i(ri,Rjo)+He-i(ri,6Ri) The New many body Hamiltonian in this approximation is: H=He(r)+H(ri,Rio)+H(R)+H-i (ri,5R)(1) (neglect the last 2 terms in band calculations)or H=HE [1st 2 terms of (1)]+HI [2nd 2 terms of (1)] HE=Electron Part(gives the energy bands),H=Ion Part(gives the phonons) For electronic properties(bands),this allows us to focus on the electronicpartof the many-body Hamiltonian:He=He(ri)+Hi(ri,Rjo) H(ri)=electron kinetic energy+electron-electron(Coulomb)repulsion H-i(ri,Rio)=electron(Coulomb)attractionto the stationary ions
The Born-Oppenheimer (Adiabatic) Approximation • Write the vibrating ion coordinates as Rj = Rjo + δRj , Rjo = equilibrium ion positions, δRj = deviation from the equilibrium positions • The many body electron-ion Hamiltonian is (schematic!): He-i ~ = He-i (ri ,Rjo) + He-i (ri ,δRj ) • The New many body Hamiltonian in this approximation is: H = He (ri ) + He-i (ri ,Rjo) + Hi (Rj ) + He-i (ri ,δRj ) (1) (neglect the last 2 terms in band calculations) or H= HE [1st 2 terms of (1)] + HI [2nd 2 terms of (1)] HE = Electron Part (gives the energy bands), HI = Ion Part (gives the phonons) In the ion Hamiltonian, Hi , the electrons can be treated in an average way! For electronic properties (bands), this allows us to focus on the electronic part of the many-body Hamiltonian:HE = He (ri ) + He-i (ri ,Rjo) He (ri ) = electron kinetic energy + electron-electron (Coulomb) repulsion He-i (ri ,Rjo) = electron (Coulomb) attraction to the stationary ions

HE =He(ri)+He-i(ri,Rjo) HE=∑p)2/(2m)+()∑i∑ir[e2Ir-rrl-∑∑lZ2Irr-Ro(i≠i') >Replace each electron momentum with its quantumoperator: Pi=-ihVi(everywhere in He!) Approximation #3 The Mean Field or One Electron Approximation For He:Make the Mean Field Approximation:Every electron experiences the same average potential due to coulomb repulsion plus coulomb attraction. The many electron Hamiltonian is replaced by a one electron Hamiltonian: Hie=(p)2/(2m)+V(r),p=-ihV In general,this is still a very complex,highly computational problem! V(r)=Effective Potential=Average potential ofone electron interacting with all others+all the ions V(r)is periodic has the lattice symmetry! Given V(r),solve the One Electron Schrodinger Equation:Hlewn=Enwn n=eigenvalue label(band index),wn=one electron wavefunction
Approximation #3 The Mean Field or One Electron Approximation For HE: Make the Mean Field Approximation: Every electron experiences the same average potential due to coulomb repulsion plus coulomb attraction. The many electron Hamiltonian is replaced by a one electron Hamiltonian: H1e = (p)2 /(2mo ) + V(r), p -iħ V(r) Effective Potential Average potential of one electron interacting with all others + all the ions V(r) is periodic & has the lattice symmetry! Given V(r), solve the One Electron Schrödinger Equation: H1eψn = Enψn n = eigenvalue label (band index) , ψn = one electron wavefunction HE = He (ri ) + He-i (ri ,Rjo) HE = ∑i (pi ) 2 /(2mi ) + (½)∑i∑i´ [e2 /|ri -ri´ |] - ∑i∑ j [Zje 2 /|ri -Rjo|] (i i´) Replace each electron momentum with its quantum operator: pi -iħi (everywhere in HE!) In general, this is still a very complex, highly computational problem!

The One-electron Approximation What about spin effects?These are neglected in He. Spin can be included.(need the Pauli Exclusion Principle:Requires quantum field theory!) Spin-orbit coupling?This is neglected in He Spin-orbit coupling can be included.(Relativistic corrections are needed.) A Rigorous Justification:Found in Hartree Hartree-Fock theory of many electron QM.Also,in the local density approximation(LDA) Start with the 1 e Hamiltonian: Hle=-(ihV)2/(2m)+V(r) Step 1:Determine the effective periodic potential V(r) However,knowing the exact form of the effective periodic potential V(r)is itself a difficultproblem! Step 2:Solve the one-electron Schrodinger Equation:Hlewn(r)=Enwn(r) V(r)=Periodic Crystal Potentialhas all of the symmetries of the crystal lattice! Translational symmetry:most important Rotational symmetry Reflection symmetry
The One-electron Approximation What about spin effects? These are neglected in HE . Spin can be included. (need the Pauli Exclusion Principle: Requires quantum field theory!) Spin-orbit coupling? This is neglected in HE . Spin-orbit coupling can be included. (Relativistic corrections are needed.) A Rigorous Justification: Found in Hartree & Hartree-Fock theory of many electron QM. Also, in the local density approximation (LDA) Start with the 1 e- Hamiltonian: H1e = -(iħ) 2 /(2me ) + V(r) Step 1: Determine the effective periodic potential V(r) However, knowing the exact form of the effective periodic potential V(r) is itself a difficult problem! Step 2: Solve the one-electron Schrödinger Equation: H1eψn(r) = Enψn(r) V(r) Periodic Crystal Potential has all of the symmetries of the crystal lattice! Translational symmetry: most important Rotational symmetry Reflection symmetry

a point worthy of attention: scientists establish the band theory with crystalline solid and from the point vies of periodic potential at first.But the introduction of periodic potential is to simplify the problem.In fact,periodic potential is not the necessary conditions for the electronic band structure,which has been confirmed to be the same as amorphous solids,eg,electron also has a band structure. Band formation results from the interaction between atoms, and does not depend on the way of atom bonding,the arrangement of atoms with translational symmetry is not the necessary conditions for the band formation
scientists establish the band theory with crystalline solid and from the point vies of periodic potential at first. But the introduction of periodic potential is to simplify the problem. In fact, periodic potential is not the necessary conditions for the electronic band structure, which has been confirmed to be the same as amorphous solids , eg, electron also has a band structure. Band formation results from the interaction between atoms, and does not depend on the way of atom bonding, the arrangement of atoms with translational symmetry is not the necessary conditions for the band formation. a point worthy of attention:
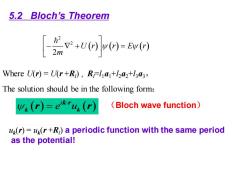
5.2 Bloch's Theorem [%+t%v=m Where U(r)=U(r+R)),RFl a+ha2+l3a3, The solution should be in the following form: (r)=ekr4(r) (Bloch wave function) (r)=u(r+R)a periodic function with the same period as the potential!
( ) ( ) ( ) 2 2 2 U r r E r m − + = Where U(r) = U(r +Rl ) , Rl=l1a1+l2a2+l3a3, The solution should be in the following form: ( ) ( ) i e u k k = k r r r (Bloch wave function) 5.2 Bloch’s Theorem uk (r) = uk (r +Rl ) a periodic function with the same period as the potential!

Recall,for a free e-,the wavefunctions have the form: yf(r)=eikr (a plane wave) A Bloch Function is the generalization of a plane wave for an e-in periodic potential.It is a plane wave modulated by a periodic function uk(r)(with the same period as V(r)) Bloch's Theorem could also be expressed as +R)=e it shows that at the corresponding point in the different cell,there is only a phase factor difference,which does not influence the value of the wave function.So,the probability of electron occurrence at the corresponding point in the different cell is the same,reflecting the crystal periodicity
Recall, for a free e-, the wavefunctions have the form: A Bloch Function is the generalization of a plane wave for an e- in periodic potential. It is a plane wave modulated by a periodic function uk (r) (with the same period as V(r)). Bloch’s Theorem could also be expressed as ( ) ( ) ( ) i k Rn n k k r R e r + = it shows that at the corresponding point in the different cell, there is only a phase factor difference, which does not influence the value of the wave function. So, the probability of electron occurrence at the corresponding point in the different cell is the same, reflecting the crystal periodicity
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 4 lattice dynamics and lattice capacity 4.5 Heat capacity of the lattice.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 4 lattice dynamics and lattice capacity 4.1 Normal Modes of Vibration 4.2 Density of States 4.3 Harmonic approximation and normal mode coordinates 4.4 phonon.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 3 Interatomic Bonding 3.1 General crystal binding 3.2 typical binding.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 3 Interatomic Bonding 3(Supplement - Rules of crystal binding).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 3 Interatomic Bonding 3.3 The cohesive energy for ionic crystal.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 2 Wave Diffraction & the Reciprocal Lattice(2.1-2.5).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 2 Wave Diffraction & the Reciprocal Lattice 2.6 X-Ray Diffraction Methods 2.7 Applications of XRD.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 2 Wave Diffraction & the Reciprocal Lattice(supplement - Review & Overview of X-Rays).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 1 Crystal Structure 1.9 Elements of Symmetry 1.10 Space groups 1.11 7 crystal system and 14 Bravis Lattice.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 1 Crystal Structure(Supplement - microstructure and crystal system).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Introduction.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 1 Crystal Structure 1.1 Elementary Crystallography 1.2 Crystal Structure ≡ Lattice + Basis 1.3 Lattice Translation Vectors 1.4 Non-Bravais Lattices 1.5 Wigner-Seitz Method.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 1 Crystal Structure 1.6 typical crystal structure.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 1 Crystal Structure 1.7 Lattice Sites in a Cubic Unit Cell 1.8 crystal plane and miller index.ppt
- 《固体物理学》课程教学资源(参考资料)词汇汉英对照.pdf
- 《固体物理学》课程授课教案(讲义)第五章 能带理论.pdf
- 《固体物理学》课程授课教案(讲义)第一章 晶体结构.pdf
- 《固体物理学》课程授课教案(讲义)第三章 晶体的结合.pdf
- 《固体物理学》课程授课教案(讲义)第四章 晶格振动.pdf
- 《固体物理学》课程授课教案(讲义)第二章 晶体结构的测定.pdf
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 4 lattice dynamics and lattice capacity 4.6 Anharmonic Effects 4.7 Equation of states for Lattice 4.8 Experimental methods for the determinations of lattice vibration spectroscopy.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 4 lattice dynamics and lattice capacity 4(supplement - Thermal Expansion of Glass).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 5 band theory 5.5 The symmetry of bands.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 5 band theory 5.3 Nearly Free Electron 5.4 BZ and energy bands.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 5 band theory 5.4 Tight Binding Approximation(TBA).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 6 Electron motion in the crystal 6.1 The quasi-classical description of Bloch electrons 6.2. Electron quasi-momentum 6.3 The accelerated velocity and effective mass.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 6 Electron motion in the crystal 6(supplement - a more concise description of quasi-momentum).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 6 Electron motion in the crystal 6.4 The electron motion in a constant electric field 6.5 Band structure of conductor, insulator and semiconductor.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 7 The electronic theory of metal(supplement - Sommerfield expansion).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 7 The electronic theory of metal 7.1 Fermi statistics and the heat capacity of electron 7.2 Work function and contact potential.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 5 band theory 5.6 Density of states(DOS)and Fermi surface 5.7 the electrons in the crystal 5.8 the experimental results for DOS.ppt
- 《近代物理实验》课程教学大纲.doc
- 《近代物理实验》课程教学资源(PPT课件讲稿,共五部分).ppt
- 《工程光学》课程教学大纲 Engineering Optics.doc
- 安徽大学:《工程光学》课程授课教案(讲义,共十三章,授课教师:李桂华).doc
- 《工程光学》课程实验指导书(共六个实验).doc
- 《量子力学》课程教学课件(讲稿)Chapter 4 态和力学量的表象 The representation for the states and dynamical variable.pdf
- 《量子力学》课程教学课件(讲稿)Chapter 1 绪论 Quantum mechanism(量子力学的诞生).pdf
- 《量子力学》课程教学课件(讲稿)Chapter 2 波函数和薛定谔方程 The wave function and Schrödinger Equation.pdf
- 《量子力学》课程教学课件(讲稿)Chapter 3 量子力学中的力学量 The Dynamical variable in Quantum Mechanism.pdf
