《固体物理学》课程教学课件(PPT讲稿)Chapter 3 Interatomic Bonding 3.1 General crystal binding 3.2 typical binding

3.Interatomic Bonding Objectives At the end of this Chapter,you should: 1.Be able to identify the bonding type in the solids. 2.Be able to calculate the cohesive energy and the related physics parameters for ionic crystals and covalent crystals 3.Understand the concept of Madelung constant
3. Interatomic Bonding Objectives At the end of this Chapter, you should: 1.Be able to identify the bonding type in the solids. 2. Be able to calculate the cohesive energy and the related physics parameters for ionic crystals and covalent crystals 3. Understand the concept of Madelung constant

3.1 General crystal binding The stable bonding arrangement:the spatial configuration of positive ion cores outer electrons has a smaller QUANTUM MECHANICAL TOTAL ENERGY than any other configuration of these particles The Cohesive Energy:the energy difference of the configuration of atoms compared with that of the isolated atoms.~0.1 eV/atom for solids ~7 eV/atom or greater in some covalent some ionic compounds some metals. Each bonding mechanism between the atoms in a solid is a result of the electrostatic interaction between the nuclei the electrons
3.1 General crystal binding The stable bonding arrangement: the spatial configuration of positive ion cores & outer electrons has a smaller QUANTUM MECHANICAL TOTAL ENERGY than any other configuration of these particles The Cohesive Energy: the energy difference of the configuration of atoms compared with that of the isolated atoms. ~ 0.1 eV/atom for solids ~ 7 eV/atom or greater in some covalent & some ionic compounds & some metals. Each bonding mechanism between the atoms in a solid is a result of the electrostatic interaction between the nuclei & the electrons

The energy of a crystal is lower than that of the free atoms by an amount equal to the energy required to pull the crystal apart into a set of free atoms.This is called the crystal Binding (Cohesive)Enerqy. En=EN-U Uo:total energy when crystal is at OK,EN:the sum of energy for N free ionics.E(cohesive energy,binding energy):at OK, the energy needed to separate all the neutral free atoms from the crystal。Take EN=O, E6=-U
The energy of a crystal is lower than that of the free atoms by an amount equal to the energy required to pull the crystal apart into a set of free atoms. This is called the crystal Binding (Cohesive) Energy. E E U b N = − 0 E U b = − 0 U0 : total energy when crystal is at 0K,EN: the sum of energy for N free ionics. Eb (cohesive energy, binding energy) :at 0K, the energy needed to separate all the neutral free atoms from the crystal。Take EN =0
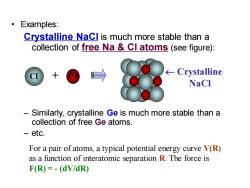
Examples: Crystalline Nacl is much more stable than a collection of free Na Cl atoms (see figure): Crystalline NaCl Similarly,crystalline Ge is much more stable than a collection of free Ge atoms. -etc. For a pair of atoms,a typical potential energy curve V(R) as a function of interatomic separation R.The force is F(R)=-(dV/dR)
• Examples: Crystalline NaCl is much more stable than a collection of free Na & Cl atoms (see figure): – Similarly, crystalline Ge is much more stable than a collection of free Ge atoms. – etc. Na Crystalline NaCl Cl + For a pair of atoms, a typical potential energy curve V(R) as a function of interatomic separation R. The force is F(R) = - (dV/dR)
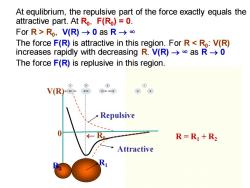
At equlibrium,the repulsive part of the force exactly equals the attractive part.At Ro,F(Ro)=0. ForR>Ro,V(R)-0asR→∞ The force F(R)is attractive in this region.For Ras R->0 The force F(R)is replusive in this region. V(R Repulsive 0 R=R1+R2 Attractive
R2 R1 V(R) 0 R0 Repulsive Attractive At equlibrium, the repulsive part of the force exactly equals the attractive part. At R0 , F(R0 ) = 0. For R > R0 , V(R) → 0 as R → ∞ The force F(R) is attractive in this region. For R < R0 : V(R) increases rapidly with decreasing R. V(R) → ∞ as R → 0 The force F(R) is replusive in this region. R = R1 + R2

Equations: u(r)= a,B.m.n>o determined by the experiment attractive repulsive 鞋≤ If stable: f(r)=- ou(r) =0 u(r) 8r2 >0 The equilibrium ou(r)=m- a b *0 nb m+1 -n- n-m Or 6=( ma The energy at ()=- (1-) m,≤习 stable point Another of-used expression (r)=- +Ae
If stable: 0 0 2 2 ( ) ( ) 0 ( ) 0 r r u r f r r u r r = − = The equilibrium 0 0 1 ( ) (1 ) m m u r r n The energy at = − − stable point 1 1 ( ) 0 m n u r a b m n r r r + + = − = 1 0 ( )n m nb r ma − = Another of-used expression
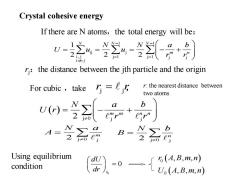
Crystal cohesive energy If there are N atoms,the total energy will be: w之4器=÷】 2 r:the distance between the jth particle and the origin For cubic,take r:the nearest distance between two atoms b A- B= 2o Using equilibrium 6(A,B,m,n) condition
If there are N atoms,the total energy will be: 1 1 ij j j 1 j 1 j j 1 2 2 2 N N N m n N N a b U u u r r − − = = = = = − + i,j i≠j ( ) 2 j 0 j j m m n n N a b U r r r = − + j j For cubic ,take r r = , Crystal cohesive energy rj:the distance between the jth particle and the origin r: the nearest distance between two atoms 2 j 0 j m N a A = 2 j 0 j n N b B = 0 0 r dU dr = Using equilibrium condition ( ) ( ) 0 0 , , , , , , r A B m n U A B m n

Elastic properties for crystals: dp thermodynamically K= k(ar) P=-KA Ps aU a"U av K-Voav2 The first term is zero at equilibrium Classically understanding Exterior pressure Hook's law:Volumetric modulus S 6(A,B,m,n) U (A,B,m,n) K=Vo( a U Elastic modulus
Elastic properties for crystals: 1 ( )T P K V V = = − thermodynamically V P K V = − 0 0 2 2 V V U U U P V V V V = − = − − 0 2 0 2 ( )V U K V V = dp K dV V = − The first term is zero at equilibrium Classically understanding Hook’s law: f dx K S x = V P K V = − Exterior pressure Volumetric modulus 0 0 r dU dr = ( ) ( ) 0 0 , , , , , , r A B m n U A B m n 0 2 0 2 ( )V U K V V = Elastic modulus
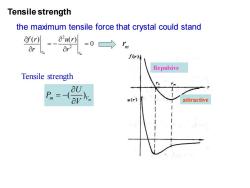
Tensile strength the maximum tensile force that crystal could stand f四=-aw =01m f(r) Repulsive Tensile strength aU Pm= av attractive
Tensile strength the maximum tensile force that crystal could stand ( ) m Vm U P V = − m r Repulsive attractive 2 2 ( ) ( ) 0 m m r r f r u r r r = − = m r Tensile strength

Basic types for crystals binding Attractive force:Coulomb interaction between opposite charges Repulsive force:Coulomb interaction between identical charges and Pauli exclusion law. Type of Form of Unit Solid Particles Forces Between Particles Properties Examples Molecular Atoms or London dispersion Fairly soft,low to moderately Argon,Ar;methane, molecules forces,dipole-dipole high melting point,poor CH4;sucrose, forces,hydrogen thermal and electrical C2H2O1:Dry bonds conduction IceTM,CO2 Covalent- Atoms connected Covalent bonds Very hard,very high melting Diamond,C;quartz, network in a network of point,often poor thermal SiO2 covalent bonds and electrical conduction Ionic Positive and Electrostatic Hard and brittle,high melting Typical salts-for negative ions attractions point,poor thermal and example,NaCl. electrical conduction Ca(NO3)2 Metallic Atoms Metallic bonds Soft to very hard,low to All metallic very high melting point, elements-for excellent thermal and example,Cu,Fe, electrical conduction, Al,Pt malleable and ductile In fact,bonding is complex and often composed of mixed
Basic types for crystals binding Attractive force: Coulomb interaction between opposite charges Repulsive force: Coulomb interaction between identical charges and Pauli exclusion law. In fact, bonding is complex and often composed of mixed
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 3 Interatomic Bonding 3(Supplement - Rules of crystal binding).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 3 Interatomic Bonding 3.3 The cohesive energy for ionic crystal.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 2 Wave Diffraction & the Reciprocal Lattice(2.1-2.5).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 2 Wave Diffraction & the Reciprocal Lattice 2.6 X-Ray Diffraction Methods 2.7 Applications of XRD.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 2 Wave Diffraction & the Reciprocal Lattice(supplement - Review & Overview of X-Rays).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 1 Crystal Structure 1.9 Elements of Symmetry 1.10 Space groups 1.11 7 crystal system and 14 Bravis Lattice.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 1 Crystal Structure(Supplement - microstructure and crystal system).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Introduction.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 1 Crystal Structure 1.1 Elementary Crystallography 1.2 Crystal Structure ≡ Lattice + Basis 1.3 Lattice Translation Vectors 1.4 Non-Bravais Lattices 1.5 Wigner-Seitz Method.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 1 Crystal Structure 1.6 typical crystal structure.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 1 Crystal Structure 1.7 Lattice Sites in a Cubic Unit Cell 1.8 crystal plane and miller index.ppt
- 《固体物理学》课程教学资源(参考资料)词汇汉英对照.pdf
- 《固体物理学》课程授课教案(讲义)第五章 能带理论.pdf
- 《固体物理学》课程授课教案(讲义)第一章 晶体结构.pdf
- 《固体物理学》课程授课教案(讲义)第三章 晶体的结合.pdf
- 《固体物理学》课程授课教案(讲义)第四章 晶格振动.pdf
- 《固体物理学》课程授课教案(讲义)第二章 晶体结构的测定.pdf
- 《固体物理学》课程授课教案(讲义)晶体能带结构理解电导特性.doc
- 《固体物理学》课程授课教案(讲义)第六章 固体电子论基础.pdf
- 《固体物理学》课程授课教案(讲义)紧束缚近似理论研究性教学教案设计.doc
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 4 lattice dynamics and lattice capacity 4.1 Normal Modes of Vibration 4.2 Density of States 4.3 Harmonic approximation and normal mode coordinates 4.4 phonon.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 4 lattice dynamics and lattice capacity 4.5 Heat capacity of the lattice.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 5 band theory 5.1 general background and approximations 5.2 Bloch’s Theorem.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 4 lattice dynamics and lattice capacity 4.6 Anharmonic Effects 4.7 Equation of states for Lattice 4.8 Experimental methods for the determinations of lattice vibration spectroscopy.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 4 lattice dynamics and lattice capacity 4(supplement - Thermal Expansion of Glass).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 5 band theory 5.5 The symmetry of bands.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 5 band theory 5.3 Nearly Free Electron 5.4 BZ and energy bands.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 5 band theory 5.4 Tight Binding Approximation(TBA).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 6 Electron motion in the crystal 6.1 The quasi-classical description of Bloch electrons 6.2. Electron quasi-momentum 6.3 The accelerated velocity and effective mass.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 6 Electron motion in the crystal 6(supplement - a more concise description of quasi-momentum).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 6 Electron motion in the crystal 6.4 The electron motion in a constant electric field 6.5 Band structure of conductor, insulator and semiconductor.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 7 The electronic theory of metal(supplement - Sommerfield expansion).ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 7 The electronic theory of metal 7.1 Fermi statistics and the heat capacity of electron 7.2 Work function and contact potential.ppt
- 《固体物理学》课程教学课件(PPT讲稿)Chapter 5 band theory 5.6 Density of states(DOS)and Fermi surface 5.7 the electrons in the crystal 5.8 the experimental results for DOS.ppt
- 《近代物理实验》课程教学大纲.doc
- 《近代物理实验》课程教学资源(PPT课件讲稿,共五部分).ppt
- 《工程光学》课程教学大纲 Engineering Optics.doc
- 安徽大学:《工程光学》课程授课教案(讲义,共十三章,授课教师:李桂华).doc
- 《工程光学》课程实验指导书(共六个实验).doc
- 《量子力学》课程教学课件(讲稿)Chapter 4 态和力学量的表象 The representation for the states and dynamical variable.pdf
