《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 06 An Overview of Organic Reactions

CNGNGE JOHN MCMURRY CHAPTER 6 An Overview of Organic Reactions T HI R D EDITION Organic Chemistry with Biological Applications
CHAPTER 6 An Overview of Organic Reactions

6-1 Kinds of Organic Reactions Organic chemical reactions broadly organized in two ways: 1.What kinds of reactions occur 2.How those reactions occur
Organic chemical reactions broadly organized in two ways: 1. What kinds of reactions occur 2. How those reactions occur 6-1 Kinds of Organic Reactions
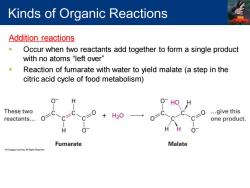
Kinds of Organic Reactions Addition reactions Occur when two reactants add together to form a single product with no atoms“left over'” Reaction of fumarate with water to yield malate (a step in the citric acid cycle of food metabolism) O HO These two H20 ...give this reactants... one product. Fumarate Malate
Addition reactions ▪ Occur when two reactants add together to form a single product with no atoms “left over” ▪ Reaction of fumarate with water to yield malate (a step in the citric acid cycle of food metabolism) Kinds of Organic Reactions
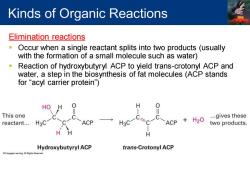
Kinds of Organic Reactions Elimination reactions Occur when a single reactant splits into two products(usually with the formation of a small molecule such as water) Reaction of hydroxybutyryl ACP to yield trans-crotonyl ACP and water,a step in the biosynthesis of fat molecules(ACP stands for "acyl carrier protein") HO H 0 This one ...gives these reactant... H3C ACP H3C =C ACP +H20 two products. H Hydroxybutyryl ACP trans-Crotonyl ACP
Elimination reactions ▪ Occur when a single reactant splits into two products (usually with the formation of a small molecule such as water) ▪ Reaction of hydroxybutyryl ACP to yield trans-crotonyl ACP and water, a step in the biosynthesis of fat molecules (ACP stands for “acyl carrier protein”) Kinds of Organic Reactions
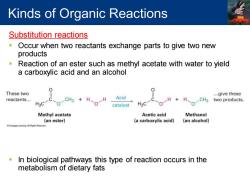
Kinds of Organic Reactions Substitution reactions 0 Occur when two reactants exchange parts to give two new products Reaction of an ester such as methyl acetate with water to yield a carboxylic acid and an alcohol 0 0 These two ...give these reactants... CH3 H H Acid H. CH3 two products. H3C catalyst H3C Methyl acetate Acetic acid Methanol (an ester) (a carboxylic acid) (an alcohol) In biological pathways this type of reaction occurs in the metabolism of dietary fats
Substitution reactions ▪ Occur when two reactants exchange parts to give two new products ▪ Reaction of an ester such as methyl acetate with water to yield a carboxylic acid and an alcohol ▪ In biological pathways this type of reaction occurs in the metabolism of dietary fats Kinds of Organic Reactions
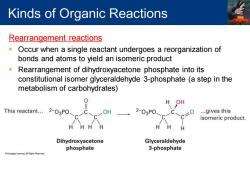
Kinds of Organic Reactions Rearrangement reactions n Occur when a single reactant undergoes a reorganization of bonds and atoms to yield an isomeric product Rearrangement of dihydroxyacetone phosphate into its constitutional isomer glyceraldehyde 3-phosphate (a step in the metabolism of carbohydrates) H OH This reactant... 2-03P0 OH 2-03P0 ...gives this isomeric product. H HH Dihydroxyacetone Glyceraldehyde phosphate 3-phosphate
Rearrangement reactions ▪ Occur when a single reactant undergoes a reorganization of bonds and atoms to yield an isomeric product ▪ Rearrangement of dihydroxyacetone phosphate into its constitutional isomer glyceraldehyde 3-phosphate (a step in the metabolism of carbohydrates) Kinds of Organic Reactions

6-2 How Organic Reactions Occur: Mechanisms Reaction Mechanism An overall description of how a reaction occurs at each stage of a chemical transformation Which bonds are broken and in what order Which bonds are formed and in what order What is the relative rate of each step A complete mechanism accounts for all reactants consumed and all products formed
Reaction Mechanism ▪ An overall description of how a reaction occurs at each stage of a chemical transformation ▪ Which bonds are broken and in what order ▪ Which bonds are formed and in what order ▪ What is the relative rate of each step ▪ A complete mechanism accounts for all reactants consumed and all products formed 6-2 How Organic Reactions Occur: Mechanisms
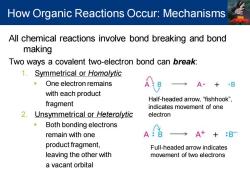
How Organic Reactions Occur:Mechanisms All chemical reactions involve bond breaking and bond making Two ways a covalent two-electron bond can break: 1.Symmetrical or Homolytic One electron remains A·+B with each product Half-headed arrow,“fishhook”, fragment indicates movement of one 2.Unsymmetrical or Heterolytic electron Both bonding electrons remain with one A:日 A++:B product fragment, Full-headed arrow indicates leaving the other with movement of two electrons a vacant orbital
All chemical reactions involve bond breaking and bond making Two ways a covalent two-electron bond can break: 1. Symmetrical or Homolytic ▪ One electron remains with each product fragment 2. Unsymmetrical or Heterolytic ▪ Both bonding electrons remain with one product fragment, leaving the other with a vacant orbital Half-headed arrow, “fishhook”, indicates movement of one electron Full-headed arrow indicates movement of two electrons How Organic Reactions Occur: Mechanisms
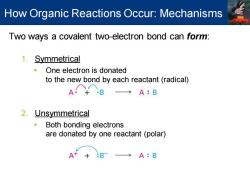
How Organic Reactions Occur:Mechanisms Two ways a covalent two-electron bond can form: 1.Symmetrical One electron is donated to the new bond by each reactant(radical) →A:B 2.Unsymmetrical Both bonding electrons are donated by one reactant(polar) A++ :BT A:B
Two ways a covalent two-electron bond can form: 1. Symmetrical ▪ One electron is donated to the new bond by each reactant (radical) 2. Unsymmetrical ▪ Both bonding electrons are donated by one reactant (polar) How Organic Reactions Occur: Mechanisms
How Organic Reactions Occur:Mechanisms Radical reaction Process that involves symmetrical bond breaking and bond making Radical (free radical) A neutral chemical species that contains an odd number of electrons and has a single,unpaired electron in one of its orbitals Polar reaction Process that involves unsymmetrical bond breaking and bond making Involve species that have an even number of electrons (have only electron pairs in their orbitals) Common in both organic and biological chemistry
Radical reaction ▪ Process that involves symmetrical bond breaking and bond making ▪ Radical (free radical) ▪ A neutral chemical species that contains an odd number of electrons and has a single, unpaired electron in one of its orbitals Polar reaction ▪ Process that involves unsymmetrical bond breaking and bond making ▪ Involve species that have an even number of electrons (have only electron pairs in their orbitals) ▪ Common in both organic and biological chemistry How Organic Reactions Occur: Mechanisms
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 05 Stereochemistry at Tetrahedral Centers.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 04 Organic Compounds - Cycloalkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 03 Organic Compounds - Alkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 02 Polar Covalent Bonds; Acids and Bases.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 01 Structure and Bonding.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Reaction Mechanism and Synthesis Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 07 Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 08 Reactions of Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 09 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 11 Structure Determination - Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 10 Structure Determination - Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 12 Organohalides - Nucleophilic Substitutions and Eliminations.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 13 Alcohols, Phenols, and Thiols; Ethers and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 14 Aldehydes and Ketones - Nucleophilic Additions Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 15 Carboxylic Acids and Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 16 Carboxylic Acid Derivatives - Nucleophilic Acyl Substitution Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 17 Carbonyl Alpha-Substitution and Condensation Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 18 Amines and Heterocycles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 19 Biomolecules - Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 20 Amino Acid Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 21 Biomolecules - Carbohydrates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 22 Carbohydrate Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 23 Biomolecules - Lipids and Their Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 24 Biomolecules - Nucleic Acids and Their Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 25 Secondary Metabolites - An Introduction to Natural Products Chemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 26 Orbitals and Organic Chemistry - Pericyclic Reactions.ppt
