《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 03 Structure and Stereochemistry of Alkanes

Organic Chemistry, ORGANIC 8th Edition CHEMISTRY L.G.Wade,Jr. Chapter 3 Lecture Structure and Stereochemistry of Alkanes G.WADE,JR Rizalia Klausmeyer Baylor University Waco,TX 2013 Pearson Education,Inc ALWAYS LEARNING PEARSON
© 2013 Pearson Education, Inc. Structure and Stereochemistry of Alkanes © 2013 Pearson Education, Inc. Chapter 3 Lecture Rizalia Klausmeyer Baylor University Waco, TX Organic Chemistry, 8 th Edition L. G. Wade, Jr
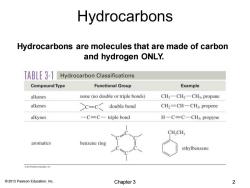
Hydrocarbons Hydrocarbons are molecules that are made of carbon and hydrogen ONLY. TABLE 3-1 Hydrocarbon Classifications Compound Type Functional Group Example alkanes none (no double or triple bonds) CH3-CH2一CH,propane alkenes C=C double bond CH2=CH一CH3,propene alkynes -C=C-triple bond H-C=C-CH3,propyne CH,CH; aromatics benzene ring ethylbenzene 201 Peon Esucationre 2013 Pearson Education,Inc. Chapter 3 2
© 2013 Pearson Education, Inc. Hydrocarbons are molecules that are made of carbon and hydrogen ONLY. Hydrocarbons Chapter 3 2

Alkanes General formula:CnHzn+2 Found in everything from natural gas to petroleum. The smaller alkanes have very low boiling points (b.p.);therefore,they are gases. CH4 C2H6 C3Ha b.p.-160℃ -89oC -420C 2013 Pearson Education,Inc. Chapter 3 3
© 2013 Pearson Education, Inc. Alkanes • General formula: CnH2n+2 • Found in everything from natural gas to petroleum. • The smaller alkanes have very low boiling points (b. p.); therefore, they are gases. CH4 C2H6 C3H8 b. p. -160 oC -89 oC -42 oC Chapter 3 3

A-Formulas and Physical Properties of the Unbranched Alkanes,Called the n-Alkanes Number Alkane of Carbons Structure Formula Density methane H-CH一H CHa -164 -183 0.55 ethane H-(C52-H C2He -89 -183 051 propane 3 H一(CH3-H C,Hy -42 -189 0.50 hutane 4 H一(CH).-H CaHio -13器 0.58 pentane H-(CH25一H CsHe 安 -130 0.63 hexane H一(CH6一H CHi 多 -95 0.66 heptane H-一(CHn一H CyHis 98 068 octane H一(aHs一H CaHu -7 0,70 monane 9 H一(CHw一H CoHzo 150 -5列 0.72 dccane 10 H-(CHo一H CioHz 174 -30 073 undecane H-(aH11一H C✉ 196 -26 074 dodecane H-(CH2)2一H Ci2H 216 -10 075 tridecane H一(aH一H CuHs 235 -5 0.76 teimadecane 4 H一(C14一H CuaHso 254 0.76 pentadecane H-(aH)s一用 CisHp 271 10 0.77 hexadecane 16 H一(Ghw一H CioHi 287 国 0,77 heptadecane 公 H一(CH1一H CnHs 303 0,76 octadeeane 体 H一(CH5)1g一H CIHs 317 2 0.76 nonadecane H一(CHe一H CioHa 330 32 0,78 cicosane 20 H-(CH)m一H CxHe 34 37 079 H一(CHe一H CxHe >450 66 081 Detvin20C.excepfo mthanedthwhse demsreven hr bolingn 2013 Pearson Education,Inc. Chapter 3 4
© 2013 Pearson Education, Inc. Chapter 3 4
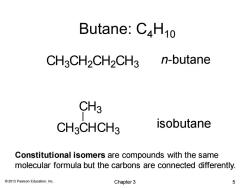
Butane:C4H10 CH3CH2CH2CH3 n-butane CH3 CH3CHCH3 isobutane Constitutional isomers are compounds with the same molecular formula but the carbons are connected differently. 2013 Pearson Education,Inc. Chapter 3 5
© 2013 Pearson Education, Inc. Butane: C4H10 Constitutional isomers are compounds with the same molecular formula but the carbons are connected differently. CH3CH2CH2CH3 CH3CHCH3 CH3 n-butane isobutane Chapter 3 5
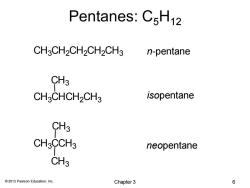
Pentanes:C5H12 CH3CH2CH2CH2CH3 n-pentane CH3 CHCHCH2CH3 isopentane CH3 CH3CCH3 neopentane CH3 2013 Pearson Education,Inc. Chapter 3 6
© 2013 Pearson Education, Inc. Pentanes: C5H12 n-pentane isopentane neopentane CH3CH2CH2CH2CH3 CH3CHCH2CH3 CH3CCH3 CH3 CH3 CH3 Chapter 3 6
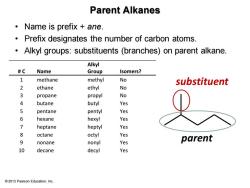
Parent Alkanes ·Name is prefix+ane. Prefix designates the number of carbon atoms. Alkyl groups:substituents (branches)on parent alkane. Alkyl #C Name Group Isomers? 1 methane methyl No substituent 2 ethane ethyl No propane propyl No 4 butane butyl Yes 5 pentane pentyl Yes 6 hexane hexyl Yes 7 heptane heptyl Yes 8 octane octyl Yes 9 nonane nonyl Yes parent 10 decane decyl Yes 2013 Pearson Education,Inc
© 2013 Pearson Education, Inc. Parent Alkanes • Name is prefix + ane. • Prefix designates the number of carbon atoms. • Alkyl groups: substituents (branches) on parent alkane. # C Name Alkyl Group Isomers? 1 methane methyl No 2 ethane ethyl No 3 propane propyl No 4 butane butyl Yes 5 pentane pentyl Yes 6 hexane hexyl Yes 7 heptane heptyl Yes 8 octane octyl Yes 9 nonane nonyl Yes 10 decane decyl Yes parent substituent

IUPAC or Systematic Names International Union of Pure and Applied Chemistry. Standard method used internationally to name compounds. Uses the longest chain of carbons as the main chain. ● Common names kept:methane,ethane, propane,and butane. 2013 Pearson Education,Inc. Chapter3 8
© 2013 Pearson Education, Inc. IUPAC or Systematic Names • International Union of Pure and Applied Chemistry. • Standard method used internationally to name compounds. • Uses the longest chain of carbons as the main chain. • Common names kept: methane, ethane, propane, and butane. Chapter 3 8

IUPAC Rules Rule 1:Find the longest continuous chain of carbon atoms,and use the name of this chain as the base name of the compound. Rule 2:Number the longest chain,beginning with the end of the chain nearest a substituent. Rule 3:Name the groups attached to the longest chain as alkyl groups.Give the location of each alkyl group by the number of the main-chain carbon atom to which it is attached. Write the alkyl groups in alphabetical order regardless of their position on the chain. 2013 Pearson Education,Inc. Chapter 3 9
© 2013 Pearson Education, Inc. IUPAC Rules • Rule 1: Find the longest continuous chain of carbon atoms, and use the name of this chain as the base name of the compound. • Rule 2: Number the longest chain, beginning with the end of the chain nearest a substituent. • Rule 3: Name the groups attached to the longest chain as alkyl groups. Give the location of each alkyl group by the number of the main-chain carbon atom to which it is attached. • Write the alkyl groups in alphabetical order regardless of their position on the chain. Chapter 3 9
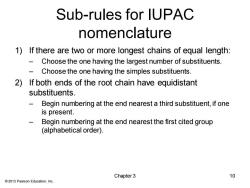
Sub-rules for IUPAC nomenclature 1)If there are two or more longest chains of equal length: Choose the one having the largest number of substituents. Choose the one having the simples substituents. 2)If both ends of the root chain have equidistant substituents. Begin numbering at the end nearest a third substituent,if one is present. Begin numbering at the end nearest the first cited group (alphabetical order). Chapter 3 10 2013 Pearson Education,Inc
© 2013 Pearson Education, Inc. Sub-rules for IUPAC nomenclature 1) If there are two or more longest chains of equal length: – Choose the one having the largest number of substituents. – Choose the one having the simples substituents. 2) If both ends of the root chain have equidistant substituents. – Begin numbering at the end nearest a third substituent, if one is present. – Begin numbering at the end nearest the first cited group (alphabetical order). Chapter 3 10
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 22 Condensations and Alpha Substitutions of Carbonyl Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 21 Part 1 - Structure and Properties of Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Reaction Mechanism and Synthesis Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 01 Structure and Bonding.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 02 Polar Covalent Bonds; Acids and Bases.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 03 Organic Compounds - Alkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 04 Organic Compounds - Cycloalkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 05 Stereochemistry at Tetrahedral Centers.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 06 An Overview of Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 07 Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 08 Reactions of Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 09 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 11 Structure Determination - Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 10 Structure Determination - Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 12 Organohalides - Nucleophilic Substitutions and Eliminations.ppt
