《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 10 Structure and Synthesis of Alcohols

Organic Chemistry,7th Edition 1.4A 0.96A L.G.Wade,Jr. 108.9 HH Chapter 10 Structure and Synthesis of Alcohols Copyright 2010 Pearson Education,Inc
Chapter 10 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Structure and Synthesis of Alcohols
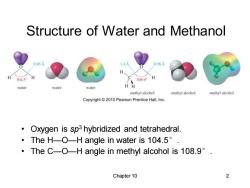
Structure of Water and Methanol 0.96A 1.4A 0.96A H 1045 H 108.9 H water water water H methyl alcohol methyl alcohol methyl alcohol Copyright2010 Pearson Prentice Hall,Inc. Oxygen is sp3 hybridized and tetrahedral. ·TheH-O-H angle in water is104.5°. ·TheC-O-H angle in methyl alcohol is108.9°. Chapter 10
Chapter 10 2 Structure of Water and Methanol • Oxygen is sp3 hybridized and tetrahedral. • The H—O—H angle in water is 104.5°. • The C—O—H angle in methyl alcohol is 108.9°

Classification of Alcohols Primary:carbon with-OH is bonded to one other carbon. Secondary:carbon with-OH is bonded to two other carbons. Tertiary:carbon with -OH is bonded to three other carbons. Aromatic (phenol):-OH is bonded to a benzene ring. Chapter 10 3
Chapter 10 3 Classification of Alcohols • Primary: carbon with —OH is bonded to one other carbon. • Secondary: carbon with —OH is bonded to two other carbons. • Tertiary: carbon with —OH is bonded to three other carbons. • Aromatic (phenol): —OH is bonded to a benzene ring
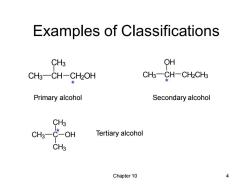
Examples of Classifications CH3 QH CH3-CH-CH2OH CH3-CH-CH2CH3 Primary alcohol Secondary alcohol CH3 CH3 C-oh Tertiary alcohol CH3 Chapter 10 4
Chapter 10 4 Examples of Classifications CH3 C CH3 CH3 * OH CH3 CH OH CH2CH3 * CH3 CH CH3 CH2OH * Primary alcohol Secondary alcohol Tertiary alcohol

IUPAC Nomenclature Find the longest carbon chain containing the carbon with the-OH group. Drop the -e from the alkane name,add -ol. Number the chain giving the-OH group the lowest number possible. Number and name all substituents and write them in alphabetical order. Chapter 10 5
Chapter 10 5 IUPAC Nomenclature • Find the longest carbon chain containing the carbon with the —OH group. • Drop the -e from the alkane name, add -ol. • Number the chain giving the —OH group the lowest number possible. • Number and name all substituents and write them in alphabetical order
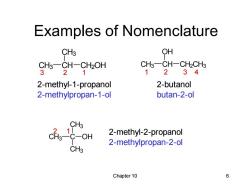
Examples of Nomenclature CH3 OH CH3一CH-CH2OH CH3-CH-CH2CH3 3 21 12 34 2-methyl-1-propanol 2-butanol 2-methylpropan-1-ol butan-2-ol CH3 1 2-methyl-2-propanol 2-methylpropan-2-ol CH3 Chapter 10 6
Chapter 10 6 Examples of Nomenclature 2-methyl-1-propanol 2-methylpropan-1-ol 2-methyl-2-propanol 2-methylpropan-2-ol 2-butanol butan-2-ol CH3 C CH3 CH3 OH CH3 CH CH3 CH2OH CH3 CH OH CH2CH3 3 2 1 1 2 3 4 2 1

Alkenols (Enols) Hydroxyl group takes precedence.Assign the carbon with the-OH the lowest number. End the name in-o/,but also specify that there is a double bond by using the ending -ene before-ol OH CH2-CHCH2CHCH3 54321 4-penten-2-ol pent-4-ene-2-ol Chapter 10 7
Chapter 10 7 Alkenols (Enols) • Hydroxyl group takes precedence. Assign the carbon with the —OH the lowest number. • End the name in –ol, but also specify that there is a double bond by using the ending –ene before -ol 4-penten-2-ol pent-4-ene-2-ol CH2 CHCH2CHCH3 OH 5 4 3 2 1

Naming Priority Highest ranking 1.Acids 2.Esters 3.Aldehydes 4.Ketones 5.Alcohols 6.Amines 7. Alkenes 8.Alkynes 9.Alkanes 10.Ethers Lowest ranking 11.Halides Chapter 10 8
Chapter 10 8 Naming Priority 1. Acids 2. Esters 3. Aldehydes 4. Ketones 5. Alcohols 6. Amines 7. Alkenes 8. Alkynes 9. Alkanes 10. Ethers 11. Halides Highest ranking Lowest ranking
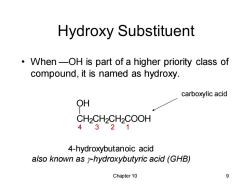
Hydroxy Substituent When-OH is part of a higher priority class of compound,it is named as hydroxy. carboxylic acid OH CH2CH2CH2COOH 4321 4-hydroxybutanoic acid also known as y-hydroxybutyric acid (GHB) Chapter 10 9
Chapter 10 9 Hydroxy Substituent • When —OH is part of a higher priority class of compound, it is named as hydroxy. 4-hydroxybutanoic acid also known as g-hydroxybutyric acid (GHB) CH2CH2CH2COOH OH carboxylic acid 4 3 2 1

Common Names Alcohol can be named as alkyl alcohol. Useful only for small alkyl groups. CH3 QH CH3-CH-CH2OH CH3-CH-CH2CH3 isobutyl alcohol sec-butyl alcohol Chapter 10 10
Chapter 10 10 Common Names • Alcohol can be named as alkyl alcohol. • Useful only for small alkyl groups. isobutyl alcohol sec-butyl alcohol CH3 CH CH3 CH2OH CH3 CH OH CH2CH3
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 21 Part 1 - Structure and Properties of Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 22 Condensations and Alpha Substitutions of Carbonyl Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 05 Stereochemistry.ppt
