《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules
Organic Chemistry,7th Edition L.G.Wade,Jr. Chapter 2 Structure and Properties of Organic Molecules Copyright 2010 Pearson Education,Inc
Chapter 2 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Structure and Properties of Organic Molecules
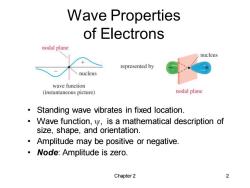
Wave Properties of Electrons nodal plane nucleus represented by nucleus wave function (instantaneous picture) nodal plane Standing wave vibrates in fixed location. Wave function,y,is a mathematical description of size,shape,and orientation. Amplitude may be positive or negative. Node:Amplitude is zero. Chapter 2 2
Chapter 2 2 Wave Properties of Electrons • Standing wave vibrates in fixed location. • Wave function, , is a mathematical description of size, shape, and orientation. • Amplitude may be positive or negative. • Node: Amplitude is zero
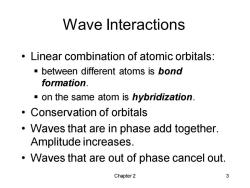
Wave Interactions Linear combination of atomic orbitals: -between different atoms is bond formation. on the same atom is hybridization. Conservation of orbitals Waves that are in phase add together. Amplitude increases. Waves that are out of phase cancel out. Chapter 2 3
Chapter 2 3 Wave Interactions • Linear combination of atomic orbitals: ▪ between different atoms is bond formation. ▪ on the same atom is hybridization. • Conservation of orbitals • Waves that are in phase add together. Amplitude increases. • Waves that are out of phase cancel out

Sigma Bonding Electron density lies between the nuclei. A bond may be formed by s-s,p-p, s-p,or hybridized orbital overlaps. The bonding molecular orbital (MO)is lower in energy than the original atomic orbitals. The antibonding MO is higher in energy than the atomic orbitals. Chapter 2 4
Chapter 2 4 Sigma Bonding • Electron density lies between the nuclei. • A bond may be formed by s—s, p—p, s—p, or hybridized orbital overlaps. • The bonding molecular orbital (MO) is lower in energy than the original atomic orbitals. • The antibonding MO is higher in energy than the atomic orbitals
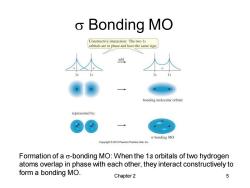
o Bonding MO Constructive interaction:The two Is orbitals are in phase and have the same sign. add bonding molecular orbital represented by: o bonding MO Pearson Prentice Hall.inc Formation of a o-bonding MO:When the 1s orbitals of two hydrogen atoms overlap in phase with each other,they interact constructively to form a bonding MO. Chapter 2 5
Chapter 2 5 s Bonding MO Formation of a s-bonding MO: When the 1s orbitals of two hydrogen atoms overlap in phase with each other, they interact constructively to form a bonding MO
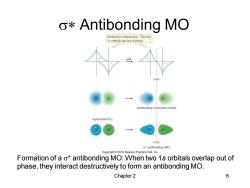
o*Antibonding MO Destructive interaction:The two Is orbitals are out of phase. antibonding molecular orbital represented by: node r本antibonding MO Copyright2010 Pearson Prentice Hall,Inc. Formation of a o*antibonding MO:When two 1s orbitals overlap out of phase,they interact destructively to form an antibonding MO. Chapter 2 6
Chapter 2 6 s* Antibonding MO Formation of a s* antibonding MO: When two 1s orbitals overlap out of phase, they interact destructively to form an antibonding MO
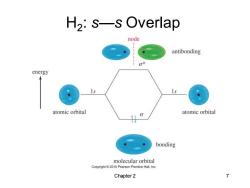
H2:s-s Overlap node antibonding energy 1s atomic orbital atomic orbital bonding molecular orbital Copyright2010 Pearson Prentice Hall,Inc. Chapter 2 7
Chapter 2 7 H2 : s—s Overlap
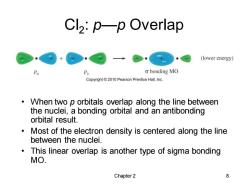
Cl2:p-p Overlap (lower energy) P Px o bonding MO Copyright 2010 Pearson Prentice Hall,Inc. When two p orbitals overlap along the line between the nuclei,a bonding orbital and an antibonding orbital result. Most of the electron density is centered along the line between the nuclei. This linear overlap is another type of sigma bonding MO. Chapter 2 8
Chapter 2 8 Cl2 : p—p Overlap • When two p orbitals overlap along the line between the nuclei, a bonding orbital and an antibonding orbital result. • Most of the electron density is centered along the line between the nuclei. • This linear overlap is another type of sigma bonding MO
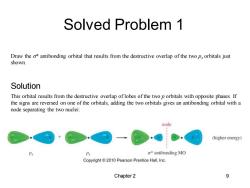
Solved Problem 1 Draw the o*antibonding orbital that results from the destructive overlap of the two px orbitals just shown. Solution This orbital results from the destructive overlap of lobes of the two p orbitals with opposite phases.If the signs are reversed on one of the orbitals,adding the two orbitals gives an antibonding orbital with a node separating the two nuclei: node (higher energy) Px o*antibonding MO Copyright 2010 Pearson Prentice Hall,Inc. Chapter 2 9
Solved Problem 1 Chapter 2 9 Draw the s* antibonding orbital that results from the destructive overlap of the two px orbitals just shown. This orbital results from the destructive overlap of lobes of the two p orbitals with opposite phases. If the signs are reversed on one of the orbitals, adding the two orbitals gives an antibonding orbital with a node separating the two nuclei: Solution
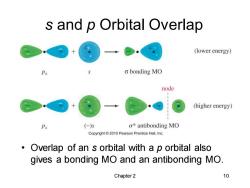
s and p Orbital Overlap (lower energy) o bonding MO node (higher energy) Px (-)5 σ*antibonding MO Copyright2010 Pearson Prentice Hall.Inc. Overlap of an s orbital with a p orbital also gives a bonding MO and an antibonding MO. Chapter 2 10
Chapter 2 10 s and p Orbital Overlap • Overlap of an s orbital with a p orbital also gives a bonding MO and an antibonding MO
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 21 Part 1 - Structure and Properties of Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 22 Condensations and Alpha Substitutions of Carbonyl Compounds.ppt
