《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 09 Alkynes

Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 9 Alkynes Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
Chapter 9 Alkynes Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr

H Introduction Alkynes contain a triple bond. General formula is CnH2n-2 Two elements of unsaturation for each triple bond. Some reactions are like alkenes: addition and oxidation. Some reactions are specific to alkynes. 三> Chapter9 2
Chapter 9 2 Introduction • Alkynes contain a triple bond. • General formula is CnH2n-2 • Two elements of unsaturation for each triple bond. • Some reactions are like alkenes: addition and oxidation. • Some reactions are specific to alkynes. =>

Nomenclature:IUPAC Find the longest chain containing the triple bond. Change -ane ending to -yne. Number the chain,starting at the end closest to the triple bond. Give branches or other substituents a number to locate their position. => Chapter 9 3
Chapter 9 3 Nomenclature: IUPAC • Find the longest chain containing the triple bond. • Change -ane ending to -yne. • Number the chain, starting at the end closest to the triple bond. • Give branches or other substituents a number to locate their position. =>

H Name these: CH3一C≡CH propyne CH一C=C-CH一CH一Br 5-bromo-2-pentyne CH3 CH3 CH3一CH一CH一C=C-CH-CH 2,6-dime thyl-3-he ptyne > Chapter 9 4
Chapter 9 4 Name these: CH3 CH CH3 CH2 C C CH CH3 CH3 CH3 C C CH2 CH2 Br CH3 C CH propyne 5-bromo-2-pentyne 2,6-dimethyl-3-heptyne =>

Additional Functional H Groups All other functional groups,except ethers and halides have a higher priority than alkynes. For a complete list of naming priorities, look inside the back cover of your text. => Chapter 9 5
Chapter 9 5 Additional Functional Groups • All other functional groups, except ethers and halides have a higher priority than alkynes. • For a complete list of naming priorities, look inside the back cover of your text. =>
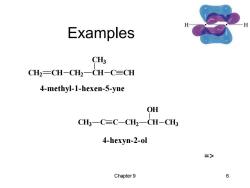
Examples C CH2-CH-CH2-CH-C=CH 4-methyl-1-hexen-5-yne OH CH3一C=C-CH2一CH-CH3 4-hexyn-2-ol 3> Chapter 9 6
Chapter 9 6 Examples CH2 CH CH2 CH CH3 C CH 4-methyl-1-hexen-5-yne CH3 C C CH2 CH OH CH3 4-hexyn-2-ol =>

Common Names Named as substituted acetylene. CH3-C=CH methylacetylene CH3 CH3 CH;-CH-CH2-C=CCH-CH3 isobutylisopropylacetylene Chapter 9
Chapter 9 7 Common Names Named as substituted acetylene. CH3 C CH methylacetylene CH3 CH CH3 CH2 C C CH CH3 CH3 isobutylisopropylacetylene =>

Physical Properties Nonpolar,insoluble in water. Soluble in most organic solvents. Boiling points similar to alkane of same size. Less dense than water. Up to 4 carbons,gas at room temperature. 二> Chapter9 8
Chapter 9 8 Physical Properties • Nonpolar, insoluble in water. • Soluble in most organic solvents. • Boiling points similar to alkane of same size. • Less dense than water. • Up to 4 carbons, gas at room temperature. =>

Acetylene Acetylene is used in welding torches. In pure oxygen,temperature of flame reaches 2800C. It would violently decompose to its elements,but the cylinder on the torch contains crushed firebrick wet with acetone to moderate it. > Chapter 9 9
Chapter 9 9 Acetylene • Acetylene is used in welding torches. • In pure oxygen, temperature of flame reaches 2800C. • It would violently decompose to its elements, but the cylinder on the torch contains crushed firebrick wet with acetone to moderate it. =>
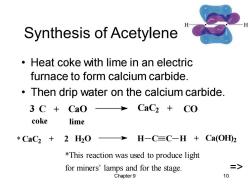
Synthesis of Acetylene Heat coke with lime in an electric furnace to form calcium carbide. Then drip water on the calcium carbide. 3 C+CaO CaC2+CO coke lime *CaC2+2H20> H-C≡C-H+Ca(OHD2 *This reaction was used to produce light for miners'lamps and for the stage => Chapter 9 10
Chapter 9 10 Synthesis of Acetylene • Heat coke with lime in an electric furnace to form calcium carbide. • Then drip water on the calcium carbide. CaC2 + 2 H2O H C C H + Ca(OH) 2 3 C + CaO CaC2 + CO coke lime *This reaction was used to produce light for miners’ lamps and for the stage. => *
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 03 Structure and Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 01 Introduction and Review(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 06 Haluros de Alquilo - Substitución Nucleofílica y Eliminación.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions.ppt
