《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 16 Aromatic Compounds

Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 16 Aromatic Compounds Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 16 Aromatic Compounds Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall Organic Chemistry, 6th Edition L. G. Wade, Jr

Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to be C6He. Other related compounds with low C:H ratios had a pleasant smell,so they were classified as aromatic. Chapter 16
Chapter 16 2 Discovery of Benzene • Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. • Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to be C6H6 . • Other related compounds with low C:H ratios had a pleasant smell, so they were classified as aromatic. =>

Kekule Structure Proposed in 1866 by Friedrich Kekule,shortly after multiple bonds were suggested. Failed to explain existence of only one isomer of 1,2-dichlorobenzene. H => Chapter 16 3
Chapter 16 3 Kekulé Structure • Proposed in 1866 by Friedrich Kekulé, shortly after multiple bonds were suggested. • Failed to explain existence of only one isomer of 1,2-dichlorobenzene. C C C C C C H H H H H H =>
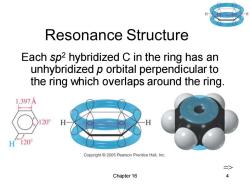
Resonance Structure Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring. 1.397A 20 H120° Copyright2005 Pearson Prentice Hall,Inc. Chapter 16
Chapter 16 4 Resonance Structure Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring. =>

Unusual Reactions ·Alkene+KMnO4→diol(addition) Benzene KMnO>no reaction. ·Alkene+Brz/CCl4→dibromide(addition) Benzene Br2/CCla>no reaction. With FeCla catalyst,Br2 reacts with benzene to form bromobenzene HBr (substitution!).Double bonds remain. > Chapter 16 5
Chapter 16 5 Unusual Reactions • Alkene + KMnO4 → diol (addition) Benzene + KMnO4 → no reaction. • Alkene + Br2 /CCl4 → dibromide (addition) Benzene + Br2 /CCl4 → no reaction. • With FeCl3 catalyst, Br2 reacts with benzene to form bromobenzene + HBr (substitution!). Double bonds remain. =>
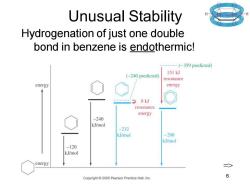
Unusual Stability Hydrogenation of just one double bond in benzene is endothermic! --…(-359 predicted) 151kJ (-240 predicted) resonance energy energy 8kJ resonance energy -240 kJ/mol -232 kJ/mol -208 kJ/mol -120 kJ/mol energy Copyright 2005 Pearson Prentice Hall,Inc. 6
Chapter 16 6 Unusual Stability Hydrogenation of just one double bond in benzene is endothermic! =>
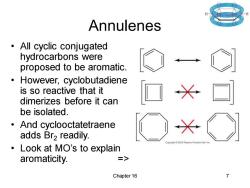
Annulenes All cyclic conjugated hydrocarbons were proposed to be aromatic. However,cyclobutadiene is so reactive that it dimerizes before it can be isolated. And cyclooctatetraene adds Br2 readily. Pea:son Prentice Hall, Look at MO's to explain aromaticity. => Chapter 16 7
Chapter 16 7 Annulenes • All cyclic conjugated hydrocarbons were proposed to be aromatic. • However, cyclobutadiene is so reactive that it dimerizes before it can be isolated. • And cyclooctatetraene adds Br2 readily. • Look at MO’s to explain aromaticity. =>

MO Rules for Benzene Six overlapping p orbitals must form six molecular orbitals. Three will be bonding,three antibonding. Lowest energy MO will have all bonding interactions,no nodes. As energy of MO increases,the number of nodes increases. 二> Chapter 16 8
Chapter 16 8 MO Rules for Benzene • Six overlapping p orbitals must form six molecular orbitals. • Three will be bonding, three antibonding. • Lowest energy MO will have all bonding interactions, no nodes. • As energy of MO increases, the number of nodes increases. =>
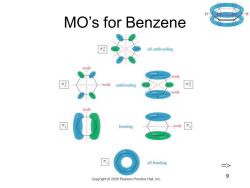
MO's for Benzene all antibonding node .node antibonding node bonding ode all bonding => 9 Copyright2005 Pearson Prentice Hall,Inc
Chapter 16 9 MO’s for Benzene =>
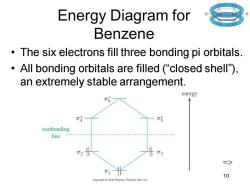
Energy Diagram for Benzene The six electrons fill three bonding pi orbitals. All bonding orbitals are filled ("closed shell). an extremely stable arrangement. energy π4 nonbonding line T3 三> 10 Copyright2005 Pearson Prentice Hall,Inc
Chapter 16 10 Energy Diagram for Benzene • The six electrons fill three bonding pi orbitals. • All bonding orbitals are filled (“closed shell”), an extremely stable arrangement. =>
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 08 Alkenes - Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 10 Alkyl Halides.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 09 Stereochemistry.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 08 Alkynes.pdf
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 06 Haluros de Alquilo - Substitución Nucleofílica y Eliminación.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 01 Introduction and Review(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 03 Structure and Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 09 Alkynes.ppt
