《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 12 Infrared Spectroscopy and Mass Spectrometry

Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 12 Infrared Spectroscopy and Mass Spectrometry Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 12 Infrared Spectroscopy and Mass Spectrometry Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall Organic Chemistry, 6th Edition L. G. Wade, Jr

Introduction Spectroscopy is an analytical technique which helps determine structure. It destroys little or no sample. The amount of light absorbed by the sample is measured as wavelength is varied. 三> Chapter 12 2
Chapter 12 2 Introduction • Spectroscopy is an analytical technique which helps determine structure. • It destroys little or no sample. • The amount of light absorbed by the sample is measured as wavelength is varied. =>
Types of Spectroscopy Infrared (IR)spectroscopy measures the bond vibration frequencies in a molecule and is used to determine the functional group. Mass spectrometry (MS)fragments the molecule and measures the masses. Nuclear magnetic resonance (NMR) spectroscopy detects signals from hydrogen atoms and can be used to distinguish isomers. Ultraviolet (UV)spectroscopy uses electron transitions to determine bonding patterns.= Chapter 12 3
Chapter 12 3 Types of Spectroscopy • Infrared (IR) spectroscopy measures the bond vibration frequencies in a molecule and is used to determine the functional group. • Mass spectrometry (MS) fragments the molecule and measures the masses. • Nuclear magnetic resonance (NMR) spectroscopy detects signals from hydrogen atoms and can be used to distinguish isomers. • Ultraviolet (UV) spectroscopy uses electron transitions to determine bonding patterns. =>

Electromagnetic Spectrum Examples:X rays,microwaves,radio waves,visible light,IR,and UV. Frequency and wavelength are inversely proportional. .c=Av,where c is the speed of light. Energy per photon hv,where h is Planck's constant,6.62 x 10-37 kJ-sec. 三> Chapter 12 4
Chapter 12 4 Electromagnetic Spectrum • Examples: X rays, microwaves, radio waves, visible light, IR, and UV. • Frequency and wavelength are inversely proportional. • c = ln, where c is the speed of light. • Energy per photon = hn, where h is Planck’s constant, 6.62 x 10-37 kJ•sec. =>
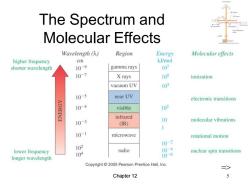
The Spectrum and Molecular Effects Wavelength(入) Region Energy Molecular effects higher frequency cm kJ/mol shorter wavelength 10-9 gamma rays 107 107 X rays 105 ionization vacuum UV 103 10-5 near UV electronic transitions 10-4 visible 102 infrared 10-3 10 molecular vibrations (IR) 1 10-1 microwave rotational motion 102 10-2 lower frequency radio 104 10-4 longer wavelength 10-6 nuclear spin transitions Copyright 2005 Pearson Prentice Hall,Inc. Chapter 12
Chapter 12 5 The Spectrum and Molecular Effects =>

The IR Region Just below red in the visible region. Wavelengths usually 2.5-25 um. More common units are wavenumbers, or cm-1,the reciprocal of the wavelength in centimeters. Wavenumbers are proportional to frequency and energy. Chapter 12 6
Chapter 12 6 The IR Region • Just below red in the visible region. • Wavelengths usually 2.5-25 mm. • More common units are wavenumbers, or cm-1 , the reciprocal of the wavelength in centimeters. • Wavenumbers are proportional to frequency and energy. =>
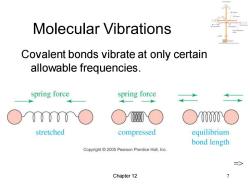
Molecular Vibrations Covalent bonds vibrate at only certain allowable frequencies. spring force spring force ○y00 stretched compressed equilibrium bond length Copyright2005 Pearson Prentice Hall,Inc. Chapter 12
Chapter 12 7 Molecular Vibrations Covalent bonds vibrate at only certain allowable frequencies. =>
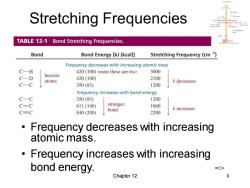
Stretching Frequencies TABLE 12-1 Bond Stretching Frequencies. Bond Bond Energy [kJ(kcal)] Stretching Frequency(cm1) Frequency decreases with increasing atomic mass C一H 420(100) 3000 C-D heavier 420(100) 2100 atoms v decreases C-C 350(83) 1200 Frequency increases with bond energy C-C 350(83) 1200 C=C 611(146) stronger 1660 bond 7 increases C=C 840(200) 2200 Frequency decreases with increasing atomic mass. Frequency increases with increasing bond energy. Chapter 12 8
Chapter 12 8 Stretching Frequencies • Frequency decreases with increasing atomic mass. • Frequency increases with increasing bond energy. =>
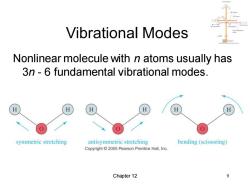
Vibrational Modes Nonlinear molecule with n atoms usually has 3n-6 fundamental vibrational modes. H symmetric stretching antisymmetric stretching bending (scissoring) Copyright 2005 Pearson Prentice Hall,Inc. Chapter 12
Chapter 12 9 Vibrational Modes Nonlinear molecule with n atoms usually has 3n - 6 fundamental vibrational modes

Fingerprint of Molecule Whole-molecule vibrations and bending vibrations are also quantized. No two molecules will give exactly the same IR spectrum (except enantiomers) Simple stretching:1600-3500 cm-1. Complex vibrations:600-1400 cm-1, called the“fingerprint region.” > Chapter 12 10
Chapter 12 10 Fingerprint of Molecule • Whole-molecule vibrations and bending vibrations are also quantized. • No two molecules will give exactly the same IR spectrum (except enantiomers). • Simple stretching: 1600-3500 cm-1 . • Complex vibrations: 600-1400 cm-1 , called the “fingerprint region.” =>
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 08 Alkenes - Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 10 Alkyl Halides.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 09 Stereochemistry.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 08 Alkynes.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 07 Alkenes - Reactions and Synthesis.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 06 Alkenes - Structure and Reactivity.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 05 An Overview of Organic Reactions.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 04 Stereochemistry of Alkanes and Cycloalkanes.pdf
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 06 Haluros de Alquilo - Substitución Nucleofílica y Eliminación.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 01 Introduction and Review(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 03 Structure and Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 05 Stereochemistry.ppt
