《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 09 Alkynes

Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 9 Alkynes Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 9 Alkynes Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall Organic Chemistry, 6th Edition L. G. Wade, Jr

Introduction Alkynes contain a triple bond. General formula is C,H2n-2. Two elements of unsaturation for each triple bond. Some reactions are like alkenes: addition and oxidation. Some reactions are specific to alkynes. > Chapter 9 2
Chapter 9 2 Introduction • Alkynes contain a triple bond. • General formula is CnH2n-2 . • Two elements of unsaturation for each triple bond. • Some reactions are like alkenes: addition and oxidation. • Some reactions are specific to alkynes. =>

Nomenclature:IUPAC Find the longest chain containing the triple bond. Change -ane ending to -yne. Number the chain,starting at the end closest to the triple bond. Give branches or other substituents a number to locate their position. 二> Chapter 9 3
Chapter 9 3 Nomenclature: IUPAC • Find the longest chain containing the triple bond. • Change -ane ending to -yne. • Number the chain, starting at the end closest to the triple bond. • Give branches or other substituents a number to locate their position. =>
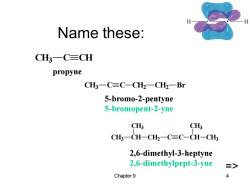
Name these: CH3一C≡CH propyne CH一C≡C-CH2一CH一Br 5-bromo-2-pentyne 5-bromopent-2-yne CHs CH3 CH一CH-CH2一C≡C-CH-CH 2,6-dimethyl-3-heptyne 2,6-dimethylpept-3-yne > Chapter 9 4
Chapter 9 4 Name these: CH3 CH CH3 CH2 C C CH CH3 CH3 CH3 C C CH2 CH2 Br CH3 C CH propyne 5-bromo-2-pentyne 5-bromopent-2-yne => 2,6-dimethyl-3-heptyne 2,6-dimethylpept-3-yne

Additional Functional Groups All other functional groups,except ethers and halides have a higher priority than alkynes. For a complete list of naming priorities, look inside the back cover of your text. => Chapter 9 5
Chapter 9 5 Additional Functional Groups • All other functional groups, except ethers and halides have a higher priority than alkynes. • For a complete list of naming priorities, look inside the back cover of your text. =>
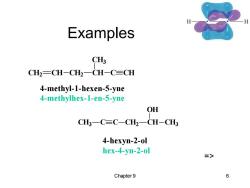
Examples C CH2=CH-CH2一CH-C=CH 4-methyl-1-hexen-5-yne 4-methylhex-1-en-5-yne OH CH3一C≡C-CH2一CH-CH3 4-hexyn-2-ol hex-4-yn-2-ol => Chapter 9 6
Chapter 9 6 Examples CH2 CH CH2 CH CH3 C CH 4-methyl-1-hexen-5-yne 4-methylhex-1-en-5-yne CH3 C C CH2 CH OH CH3 4-hexyn-2-ol hex-4-yn-2-ol =>

Common Names Named as substituted acetylene. CH3C≡CH methylacetylene (terminal alkyne) CH3 CH3 CH3一CH-CH2C=CCH-CH3 isobutylisopropylacetylene (internal alkyne) Chapter 9 7
Chapter 9 7 Common Names Named as substituted acetylene. CH3 C CH methylacetylene (terminal alkyne) CH3 CH CH3 CH2 C C CH CH3 CH3 isobutylisopropylacetylene (internal alkyne) =>

Physical Properties Nonpolar,insoluble in water. Soluble in most organic solvents. Boiling points similar to alkane of same size. Less dense than water. Up to 4 carbons,gas at room temperature. 二> Chapter 9 8
Chapter 9 8 Physical Properties • Nonpolar, insoluble in water. • Soluble in most organic solvents. • Boiling points similar to alkane of same size. • Less dense than water. • Up to 4 carbons, gas at room temperature. =>

Acetylene Acetylene is used in welding torches. In pure oxygen,temperature of flame reaches2800°C. It would violently decompose to its elements,but the cylinder on the torch contains crushed firebrick wet with acetone to moderate it. => Chapter 9 9
Chapter 9 9 Acetylene • Acetylene is used in welding torches. • In pure oxygen, temperature of flame reaches 2800C. • It would violently decompose to its elements, but the cylinder on the torch contains crushed firebrick wet with acetone to moderate it. =>
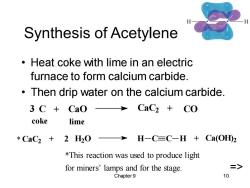
Synthesis of Acetylene Heat coke with lime in an electric furnace to form calcium carbide. Then drip water on the calcium carbide. 3 C+Cao>CaC2 CO coke lime *CaC2+2H20 H-C≡C-H+Ca(OHD2 *This reaction was used to produce light for miners'lamps and for the stage. => Chapter 9 10
Chapter 9 10 Synthesis of Acetylene • Heat coke with lime in an electric furnace to form calcium carbide. • Then drip water on the calcium carbide. CaC2 + 2 H2O H C C H + Ca(OH) 2 3 C + CaO CaC2 + CO coke lime *This reaction was used to produce light for miners’ lamps and for the stage. => *
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 08 Alkenes - Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 10 Alkyl Halides.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 09 Stereochemistry.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 08 Alkynes.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 07 Alkenes - Reactions and Synthesis.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 06 Alkenes - Structure and Reactivity.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 05 An Overview of Organic Reactions.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 04 Stereochemistry of Alkanes and Cycloalkanes.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 03 Organic Compounds - Alkanes and Cycloalkanes.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 02 Polar Covalent Bonds; Acids and Bases.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 01 Structure and Bonding.pdf
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 06 Haluros de Alquilo - Substitución Nucleofílica y Eliminación.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 01 Introduction and Review(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules(西北农林科技大学,2010).ppt
