《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 14 Ethers, Epoxides, and Sulfides

Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 14 Ethers,Epoxides, and Sulfides Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 14 Ethers, Epoxides, and Sulfides Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall Organic Chemistry, 6th Edition L. G. Wade, Jr
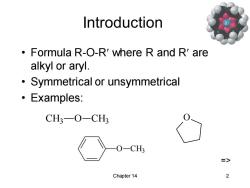
Introduction Formula R-O-R'where R and R'are alkyl or aryl. Symmetrical or unsymmetrical 。Examples: CH3-O-CH3 )-CH3 => Chapter 14 2
Chapter 14 2 Introduction • Formula R-O-R where R and R are alkyl or aryl. • Symmetrical or unsymmetrical • Examples: O CH3 CH3 O CH3 O =>
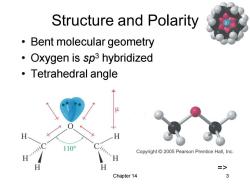
Structure and Polarity Bent molecular geometry Oxygen is sp3 hybridized 。Tetrahedral angle Copyright 2005 Pearson Prentice Hall,Inc. H => Chapter 14 3
Chapter 14 3 Structure and Polarity • Bent molecular geometry • Oxygen is sp3 hybridized • Tetrahedral angle =>
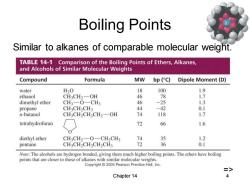
Boiling Points Similar to alkanes of comparable molecular weight. TABLE 14-1 Comparison of the Boiling Points of Ethers,Alkanes, and Alcohols of Similar Molecular Weights Compound Formula MW bp (C) Dipole Moment(D) water H20 18 100 1.9 ethanol CH3CH2-OH 46 78 1.7 dimethyl ether CH3一O-CH3 46 -25 1.3 propane CH3CH2CH3 44 -42 0.1 n-butanol CH3CH2CH2CH2一OH 74 118 1.7 tetrahydrofuran 72 66 1.6 diethyl ether CH3CH2-O-CH2CH3 74 35 1.2 pentane CH3CH2CH2CH2CH3 72 6 0.1 Note:The alcohols are hydrogen bonded.giving them much higher boiling points.The ethers have boiling points that are closer to those of alkanes with similar molecular weights. Copyright 2005 Pearson Prentice Hall,Inc. => Chapter 14 4
Chapter 14 4 Boiling Points Similar to alkanes of comparable molecular weight. =>
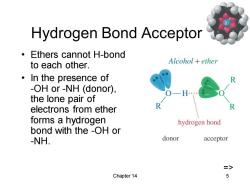
Hydrogen Bond Acceptor Ethers cannot H-bond to each other. Alcohol ether ·In the presence of -OH or-NH (donor), the lone pair of electrons from ether forms a hydrogen hydrogen bond bond with the -OH or -NH. donor acceptor => Chapter 14 5
Chapter 14 5 Hydrogen Bond Acceptor • Ethers cannot H-bond to each other. • In the presence of -OH or -NH (donor), the lone pair of electrons from ether forms a hydrogen bond with the -OH or -NH. =>
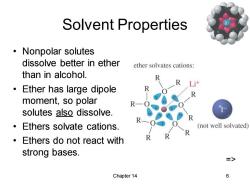
Solvent Properties ·Nonpolar solutes dissolve better in ether ether solvates cations: than in alcohol. 0 Ether has large dipole moment,so polar R solutes also dissolve. Ethers solvate cations. (not well solvated) Ethers do not react with strong bases. => Chapter 14 6
Chapter 14 6 Solvent Properties • Nonpolar solutes dissolve better in ether than in alcohol. • Ether has large dipole moment, so polar solutes also dissolve. • Ethers solvate cations. • Ethers do not react with strong bases. =>
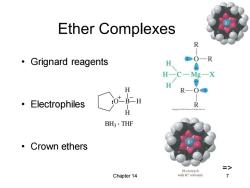
Ether Complexes R ·Grignard reagents O-R H H-C一Mg-X H H R—O8 ·Electrophiles :OtB二H R H BH3·THF ·Crown ethers 18-crown-6 Chapter 14 with K+solvated 7
Chapter 14 7 Ether Complexes • Grignard reagents • Electrophiles • Crown ethers O B H H H + _ BH3 THF =>

Common Names of Ethers ·Alkyl alkyl ether Current rule:alphabetical order Old rule:order of increasing complexity 。 Symmetrical:use dialkyl,or just alkyl. ·Examples: CH3 CH3一O-C-CH3 CH3CH2-O-CH2CH3 CH3 diethyl ether or t-butyl methyl ether or ethyl ether Chapter 14 methyl t-butyl ether a =
Chapter 14 8 Common Names of Ethers • Alkyl alkyl ether • Current rule: alphabetical order • Old rule: order of increasing complexity • Symmetrical: use dialkyl, or just alkyl. • Examples: CH3 CH2 O CH2 CH3 diethyl ether or ethyl ether CH3 O C CH3 CH3 CH3 t-butyl methyl ether or methyl t-butyl ether =>
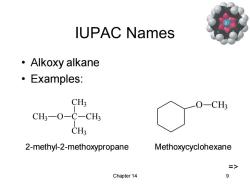
IUPAC Names ·Alkoxy alkane ·Examples: CH3 O-CH? CH3-O-C-CH3 CH3 2-methyl-2-methoxypropane Methoxycyclohexane => Chapter 14 9
Chapter 14 9 IUPAC Names • Alkoxy alkane • Examples: CH3 O C CH3 CH3 CH3 2-methyl-2-methoxypropane O CH3 Methoxycyclohexane =>
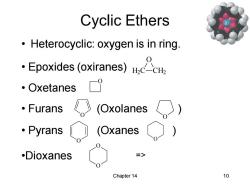
Cyclic Ethers Heterocyclic:oxygen is in ring Epoxides (oxiranes)c 。Oxetanes 。Furans 《(Oxolanes ·Pyrans (Oxanes .Dioxanes > Chapter 14 10
Chapter 14 10 Cyclic Ethers • Heterocyclic: oxygen is in ring. • Epoxides (oxiranes) H2C CH2 O • Oxetanes O • Furans (Oxolanes ) O O • Pyrans (Oxanes ) O O •Dioxanes O O =>
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 08 Alkenes - Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 10 Alkyl Halides.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 09 Stereochemistry.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 08 Alkynes.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 07 Alkenes - Reactions and Synthesis.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 06 Alkenes - Structure and Reactivity.pdf
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 06 Haluros de Alquilo - Substitución Nucleofílica y Eliminación.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 01 Introduction and Review(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 03 Structure and Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 07 Structure and Synthesis of Alkenes.ppt
