《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 13 Nuclear Magnetic Resonance Spectroscopy

Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 13 Nuclear Magnetic Resonance Spectroscopy Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 13 Nuclear Magnetic Resonance Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall Organic Chemistry, 6th Edition L. G. Wade, Jr
circulution Introduction NMR is the most powerful tool available for organic structure determination. It is used to study a wide variety of nuclei: >1H >13C >15N >19F >31P => Chapter 13
Chapter 13 2 Introduction • NMR is the most powerful tool available for organic structure determination. • It is used to study a wide variety of nuclei: ➢1H ➢13C ➢15N ➢19F ➢31P =>
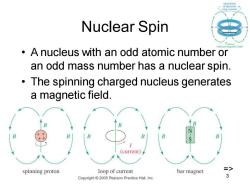
Nuclear Spin A nucleus with an odd atomic number or an odd mass number has a nuclear spin. The spinning charged nucleus generates a magnetic field. (current) spinning proton loop of current bar magnet Copyright 2005 Pearson Prentice Hall,Inc
Chapter 13 3 Nuclear Spin • A nucleus with an odd atomic number or an odd mass number has a nuclear spin. • The spinning charged nucleus generates a magnetic field. =>
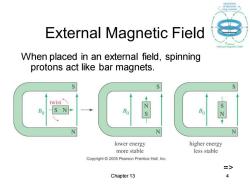
circulution External Magnetic Field When placed in an external field,spinning protons act like bar magnets. twist N S N lower energy higher energy more stable less stable Copyright 2005 Pearson Prentice Hall,Inc. => Chapter 13 4
Chapter 13 4 External Magnetic Field When placed in an external field, spinning protons act like bar magnets. =>
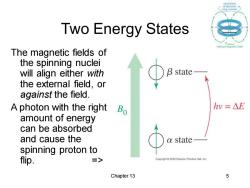
Two Energy States The magnetic fields of the spinning nuclei will align either with B state the external field,or against the field. A photon with the right Bo hv=△E amount of energy can be absorbed and cause the a state spinning proton to flip. => Copyright2005 Pearson Prentice Hall,Inc Chapter 13 5
Chapter 13 5 Two Energy States The magnetic fields of the spinning nuclei will align either with the external field, or against the field. A photon with the right amount of energy can be absorbed and cause the spinning proton to flip. =>

circulation △E and Magnet Strength Energy difference is proportional to the magnetic field strength. 。△E=hv=YhB0 2元 Gyromagnetic ratio,y,is a constant for each nucleus(26,753 s-1gauss-1 for H). In a 14,092 gauss field,a 60 MHz photon is required to flip a proton. Low energy,radio frequency. => Chapter 13 6
Chapter 13 6 E and Magnet Strength • Energy difference is proportional to the magnetic field strength. • E = h = h B0 2 • Gyromagnetic ratio, , is a constant for each nucleus (26,753 s-1gauss-1 for H). • In a 14,092 gauss field, a 60 MHz photon is required to flip a proton. • Low energy, radio frequency. =>

Magnetic Shielding If all protons absorbed the same amount of energy in a given magnetic field,not much information could be obtained. But protons are surrounded by electrons that shield them from the external field. Circulating electrons create an induced magnetic field that opposes the external magnetic field => Chapter 13 7
Chapter 13 7 Magnetic Shielding • If all protons absorbed the same amount of energy in a given magnetic field, not much information could be obtained. • But protons are surrounded by electrons that shield them from the external field. • Circulating electrons create an induced magnetic field that opposes the external magnetic field. =>
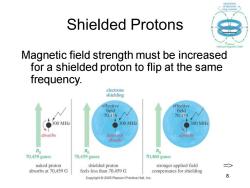
circulution Shielded Protons Magnetic field strength must be increased for a shielded proton to flip at the same frequency. electrons shielding effective effective field field 70.458 70.459 300 MHz 300MH2 300MH2 absorbs does not absorbs absorb 9 70,459 gauss 70.459 gauss 70,460 gauss naked proton shielded proton stronger applied field absorbs at 70.459 G feels less than 70,459 G compensates for shielding Copyright 2005 Pearson Prentice Hall,Inc
Chapter 13 8 Shielded Protons Magnetic field strength must be increased for a shielded proton to flip at the same frequency. =>
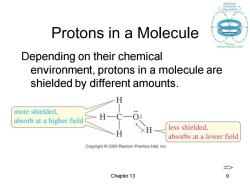
Protons in a Molecule Depending on their chemical environment,protons in a molecule are shielded by different amounts. more shielded. absorb at a higher field H less shielded, absorbs at a lower field Copyright 2005 Pearson Prentice Hall.Inc. 三> Chapter 13 9
Chapter 13 9 Protons in a Molecule Depending on their chemical environment, protons in a molecule are shielded by different amounts. =>
circulution NMR Signals The number of signals shows how many different kinds of protons are present. The location of the signals shows how shielded or deshielded the proton is. The intensity of the signal shows the number of protons of that type. Signal splitting shows the number of protons on adjacent atoms. => Chapter 13 10
Chapter 13 10 NMR Signals • The number of signals shows how many different kinds of protons are present. • The location of the signals shows how shielded or deshielded the proton is. • The intensity of the signal shows the number of protons of that type. • Signal splitting shows the number of protons on adjacent atoms. =>
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 08 Alkenes - Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 10 Alkyl Halides.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 09 Stereochemistry.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 08 Alkynes.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 07 Alkenes - Reactions and Synthesis.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 06 Alkenes - Structure and Reactivity.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 05 An Overview of Organic Reactions.pdf
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 06 Haluros de Alquilo - Substitución Nucleofílica y Eliminación.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 01 Introduction and Review(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 03 Structure and Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
