《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 03 Structure and Stereochemistry of Alkanes

Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 3 Structure and Stereochemistry of Alkanes Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 3 Structure and Stereochemistry of Alkanes Organic Chemistry, 6th Edition L. G. Wade, Jr. Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall
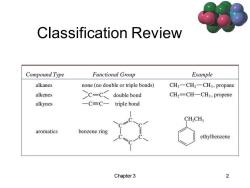
Classification Review Compound Type Functional Group Example alkanes none (no double or triple bonds) CH3一CH2-CH3,propane alkenes >c-c< double bond CH2=CH-CH3,propene alkynes 一C三C一 triple bond CHCH aromatics benzene ring ethylbenzene Chapter 3 2
Chapter 3 2 Classification Review
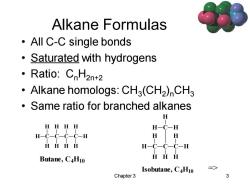
Alkane Formulas ·AllC-C single bonds Saturated with hydrogens 。Ratio::CnH2nt2 Alkane homologs:CH3(CH2)CH3 Same ratio for branched alkanes H HHHH H-C-H H-c-c-c-C-H H H HHHH H-C-C-C-H Butane,C4H10 HHH Isobutane,C4H10 => Chapter 3 3
Chapter 3 3 Alkane Formulas • All C-C single bonds • Saturated with hydrogens • Ratio: CnH2n+2 • Alkane homologs: CH3 (CH2 )nCH3 • Same ratio for branched alkanes => C H C H H H C H H H C H H H Isobutane, C4H10 C H C H H H C C H H H H H H Butane, C4H10

Common Names 。Isobutane,“isomer of butane” Isopentane,isohexane,etc.,methyl branch on next-to-last carbon in chain. Neopentane,most highly branched Five possible isomers of hexane, 18 isomers of octane and 75 for decane! Chapter 3 4
Chapter 3 4 Common Names • Isobutane, “isomer of butane” • Isopentane, isohexane, etc., methyl branch on next-to-last carbon in chain. • Neopentane, most highly branched • Five possible isomers of hexane, 18 isomers of octane and 75 for decane! =>
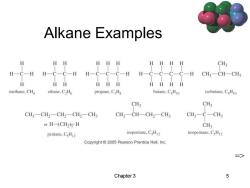
Alkane Examples HH HHHH CH3 H一C-H H-C-C-H H-C-C-C-H H-C- -C- CH3一CH-CH H HH HHH HHHH methane,CHa ethane,C2H6 propane,CHg butane,CHo isobutane,CHo CH3 CH3 CH3-CH2-CH2一CH2一CH3 CH3一CH-CH2-CH3 CH3-C-CH3 or H-fCH2)s H CH3 pentane.CsH2 isopentane.CsH2 neopentane,CsH2 Copyright 2005 Pearson Prentice Hall,Inc. 二> Chapter 3
Chapter 3 5 Alkane Examples =>

IUPAC Names 0 Find the longest continuous carbon chain. Number the carbons,starting closest to the first branch. Name the groups attached to the chain, using the carbon number as the locator. Alphabetize substituents. Use di-,tri-,etc.,for multiples of same substituent. > Chapter 3 6
Chapter 3 6 IUPAC Names • Find the longest continuous carbon chain. • Number the carbons, starting closest to the first branch. • Name the groups attached to the chain, using the carbon number as the locator. • Alphabetize substituents. • Use di-, tri-, etc., for multiples of same substituent. =>
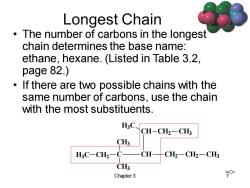
Longest Chain The number of carbons in the longest chain determines the base name: ethane,hexane.(Listed in Table 3.2, page 82.) If there are two possible chains with the same number of carbons,use the chain with the most substituents. HC、 CH-CH2-CH3 CH3 H3C-CH2-C- CH CH2-CH2-CH3 CH3 Chapter 3 >
Chapter 3 7 Longest Chain • The number of carbons in the longest chain determines the base name: ethane, hexane. (Listed in Table 3.2, page 82.) • If there are two possible chains with the same number of carbons, use the chain with the most substituents. C CH3 CH2 CH3 CH CH2 CH2 CH3 CH CH2 CH3 H3C H3C =>
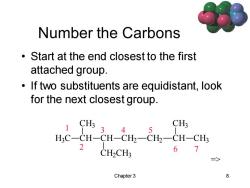
Number the Carbons Start at the end closest to the first attached group. If two substituents are equidistant,look for the next closest group. CH3 3 CH3 4 5 H3C-CH-CH-CH2一CH2一CH-CH 2 CH2CH: 67 Chapter 3 8
Chapter 3 8 Number the Carbons • Start at the end closest to the first attached group. • If two substituents are equidistant, look for the next closest group. 1 2 3 4 5 6 7 H3 C CH CH3 CH CH2 CH3 CH2 CH2 CH CH3 CH3 =>
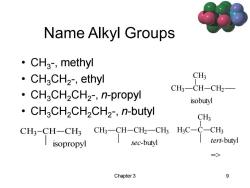
Name Alkyl Groups ·CH3,methyl ·CH3CH2,ethyl CH3 CH3一CH-CH2 ·CH3CH2CH2,n-propyl isobutyl CHaCH2CH2CH2-,n-butyl CH3 CH3-CH-CH3 CH3-CH-CH2-CH3 H;C-C-CH3 isopropyl sec-butyl tert-butyl => Chapter 3 9
Chapter 3 9 Name Alkyl Groups • CH3 -, methyl • CH3CH2 -, ethyl • CH3CH2CH2 -, n-propyl • CH3CH2CH2CH2 -, n-butyl CH3 CH CH2 CH3 sec-butyl CH3 CH CH3 CH2 isobutyl CH3 CH CH3 isopropyl H3 C C CH3 CH3 tert-butyl =>
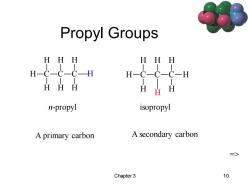
Propyl Groups HHH HHH HHH H n-propyl isopropyl A primary carbon A secondary carbon => Chapter 3 10
Chapter 3 10 Propyl Groups C H H H C H H C H H H n-propyl C H H H C H C H H H isopropyl H A primary carbon A secondary carbon =>
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 10 Alkyl Halides.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 09 Stereochemistry.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 08 Alkynes.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 07 Alkenes - Reactions and Synthesis.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 06 Alkenes - Structure and Reactivity.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 05 An Overview of Organic Reactions.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 04 Stereochemistry of Alkanes and Cycloalkanes.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 03 Organic Compounds - Alkanes and Cycloalkanes.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 02 Polar Covalent Bonds; Acids and Bases.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 01 Structure and Bonding.pdf
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2008—2009学年第二学期ORGANIC CHEMISTRY课程A卷(答案).doc
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2008—2009学年第二学期ORGANIC CHEMISTRY课程A卷(试题).doc
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2012—2013学年第2学期课程A卷(答案).pdf
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2012—2013学年第2学期课程A卷(试题).pdf
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2004年有机化学期末测试题B(双语)试题.pdf
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 08 Alkenes - Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
