《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 18 Ketones and Aldehydes

RuCt&0号 Organic Chemistry,6th Edition R L.G.Wade,Jr. Chapter 18 Ketones and Aldehydes Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 18 Ketones and Aldehydes Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall Organic Chemistry, 6th Edition L. G. Wade, Jr
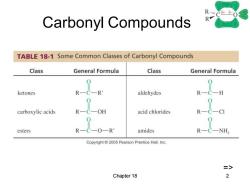
RC-0 Carbonyl Compounds R TABLE 18-1 Some Common Classes of Carbonyl Compounds Class General Formula Class General Formula ketones R-C-R aldehydes carboxylic acids R-C-OH acid chlorides 0 esters R-C-O-R amides Copyright 2005 Pearson Prentice Hall,Inc. => Chapter 18
Chapter 18 2 Carbonyl Compounds =>
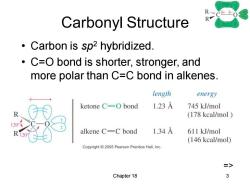
Carbonyl Structure R Carbon is sp2 hybridized. C=O bond is shorter,stronger,and more polar than C=C bond in alkenes. length energy ketone C=O bond 1.23A 745 kJ/mol (178 kcal/mol alkene C=C bond 1.34A 611 kJ/mol (146 kcal/mol) Copyright2005 Pearson Prentice Hall,Inc. Chapter 18 3
Chapter 18 3 Carbonyl Structure • Carbon is sp2 hybridized. • C=O bond is shorter, stronger, and more polar than C=C bond in alkenes. =>

RC-0 IUPAC Names R for Ketones Replace -e with -one.Indicate the position of the carbonyl with a number. Number the chain so that carbonyl carbon has the lowest number. For cyclic ketones the carbonyl carbon is assigned the number 1. Chapter 18
Chapter 18 4 IUPAC Names for Ketones • Replace -e with -one. Indicate the position of the carbonyl with a number. • Number the chain so that carbonyl carbon has the lowest number. • For cyclic ketones the carbonyl carbon is assigned the number 1. =>
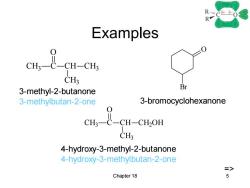
RC-0 R Examples CH-CH CH3 3-methyl-2-butanone Br 3-methylbutan-2-one 3-bromocyclohexanone 0 cus-E-CH-cton CH3 4-hydroxy-3-methyl-2-butanone 4-hydroxy-3-methylbutan-2-one 三> Chapter 18 6
Chapter 18 5 Examples CH3 C O CH CH3 CH3 O Br CH3 C O CH CH3 CH2OH 3-methyl-2-butanone 3-methylbutan-2-one 3-bromocyclohexanone 4-hydroxy-3-methyl-2-butanone 4-hydroxy-3-methylbutan-2-one =>

RC-0 R Naming Aldehydes IUPAC:Replace -e with -al. The aldehyde carbon is number 1. If-CHO is attached to a ring,use the suffix -carbaldehyde. => Chapter 18 6
Chapter 18 6 Naming Aldehydes • IUPAC: Replace -e with -al. • The aldehyde carbon is number 1. • If -CHO is attached to a ring, use the suffix -carbaldehyde. =>
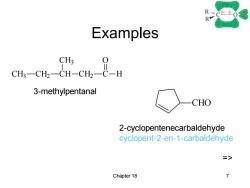
R- Examples CH3 3-methylpentanal CHO 2-cyclopentenecarbaldehyde cyclopent-2-en-1-carbaldehyde => Chapter 18 7
Chapter 18 7 Examples CH3 CH2 CH CH3 CH2 C H O CHO 3-methylpentanal 2-cyclopentenecarbaldehyde cyclopent-2-en-1-carbaldehyde =>
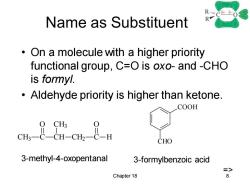
RC+-0 Name as Substituent R On a molecule with a higher priority functional group,C=O is oxo-and -CHO is formyl. Aldehyde priority is higher than ketone. COOH CHO 3-methyl-4-oxopentanal 3-formylbenzoic acid => Chapter 18 8
Chapter 18 8 Name as Substituent • On a molecule with a higher priority functional group, C=O is oxo- and -CHO is formyl. • Aldehyde priority is higher than ketone. CH3 C CH CH3 CH2 C H O O COOH CHO 3-methyl-4-oxopentanal 3-formylbenzoic acid =>
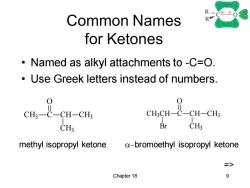
Common Names R for Ketones Named as alkyl attachments to -C=O. Use Greek letters instead of numbers. 0 em t cu-cu CHCH-C-CH-CHs CH3 Br CH3 methyl isopropyl ketone a-bromoethyl isopropyl ketone Chapter 18 9
Chapter 18 9 Common Names for Ketones • Named as alkyl attachments to -C=O. • Use Greek letters instead of numbers. CH3 C O CH CH3 CH3 CH3CH C O CH CH3 CH3 Br methyl isopropyl ketone a-bromoethyl isopropyl ketone =>
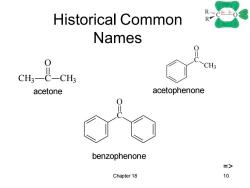
Historical Common RC-0 Names 0 >CH; acetone acetophenone benzophenone => Chapter 18 10
Chapter 18 10 Historical Common Names CH3 C O CH3 C CH3 O C O acetone acetophenone benzophenone =>
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 08 Alkenes - Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 10 Alkyl Halides.pdf
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 06 Haluros de Alquilo - Substitución Nucleofílica y Eliminación.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 01 Introduction and Review(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 03 Structure and Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 11 Reactions of Alcohols.ppt
