《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination

Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 6 Alkyl Halides:Nucleophilic Substitution and Elimination Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©20o3,Prentice Hall
Chapter 6 Alkyl Halides: Nucleophilic Substitution and Elimination Organic Chemistry, 5th Edition L. G. Wade, Jr. Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall
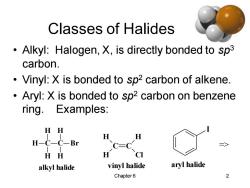
Classes of Halides Alkyl:Halogen,X,is directly bonded to sp3 carbon. Vinyl:X is bonded to sp2 carbon of alkene. Aryl:X is bonded to sp2 carbon on benzene ring.Examples: HH H H H-C一C-Br C=C HH H CI alkyl halide vinyl halide aryl halide Chapter 6 2
Chapter 6 2 Classes of Halides • Alkyl: Halogen, X, is directly bonded to sp3 carbon. • Vinyl: X is bonded to sp2 carbon of alkene. • Aryl: X is bonded to sp2 carbon on benzene ring. Examples: C H H H C H H Br alkyl halide C C H H H Cl vinyl halide I aryl halide =>
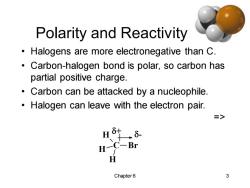
Polarity and Reactivity Halogens are more electronegative than C. Carbon-halogen bond is polar,so carbon has partial positive charge. Carbon can be attacked by a nucleophile Halogen can leave with the electron pair. => H H一 C-Br H Chapter6 3
Chapter 6 3 Polarity and Reactivity • Halogens are more electronegative than C. • Carbon-halogen bond is polar, so carbon has partial positive charge. • Carbon can be attacked by a nucleophile. • Halogen can leave with the electron pair. => C H H H Br + -

Classes of Alkyl Halides Methyl halides:only one C,CHaX Primary:C to which X is bonded has only one C-C bond. Secondary:C to which X is bonded has two C-C bonds. Tertiary:C to which X is bonded has three C-C bonds. > Chapter 6
Chapter 6 4 Classes of Alkyl Halides • Methyl halides: only one C, CH3X • Primary: C to which X is bonded has only one C-C bond. • Secondary: C to which X is bonded has two C-C bonds. • Tertiary: C to which X is bonded has three C-C bonds. =>
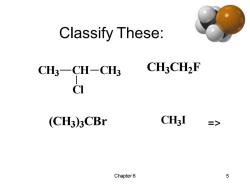
Classify These: CH3-CH-CH3 CH3CH2F CI (CH3)3CBr CH3I => Chapter6 5
Chapter 6 5 Classify These: CH3 CH CH3 Cl CH3 CH2 F (CH3) 3 CBr CH3 I =>
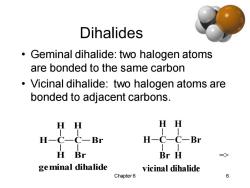
Dihalides Geminal dihalide:two halogen atoms are bonded to the same carbon Vicinal dihalide:two halogen atoms are bonded to adjacent carbons. H H HH H-C-C-Br H-C-C-Br H Br Br H ge minal dihalide vicinal dihalide Chapter 6 6
Chapter 6 6 Dihalides • Geminal dihalide: two halogen atoms are bonded to the same carbon • Vicinal dihalide: two halogen atoms are bonded to adjacent carbons. C H H H C H Br Br geminal dihalide C H H Br C H H Br vicinal dihalide =>
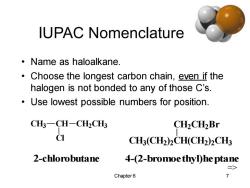
IUPAC Nomenclature ·Name as haloalkane. Choose the longest carbon chain,even if the halogen is not bonded to any of those C's. Use lowest possible numbers for position. CH3-CH-CH2CH3 CH2CH2Br C CH3(CH2)2CH(CH2)2CH3 2-chlorobutane 4-(2-bromoe thylhe ptane => Chapter 6 7
Chapter 6 7 IUPAC Nomenclature • Name as haloalkane. • Choose the longest carbon chain, even if the halogen is not bonded to any of those C’s. • Use lowest possible numbers for position. CH3 CH CH2 CH3 Cl CH3 (CH2 ) 2 CH(CH2 ) 2 CH3 CH2 CH2 Br 2-chlorobutane 4-(2-bromoethyl)heptane=>
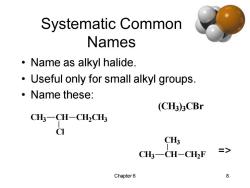
Systematic Common Names ·Name as alkyl halide. Useful only for small alkyl groups. 。Name these: (CH3)3CBr CH3-CH-CH2CH3 CI CH3 CH3-CH-CH2F > Chapter6 8
Chapter 6 8 Systematic Common Names • Name as alkyl halide. • Useful only for small alkyl groups. • Name these: CH3 CH CH2 CH3 Cl (CH3) 3 CBr CH3 CH CH3 CH2 F =>

"Trivial"Names CH2X2 called methylene halide. 。 CHX3 is a haloform. CX is carbon tetrahalide. 。Examples: >CH2Cl2 is methylene chloride >CHCIa is chloroform >CCl,is carbon tetrachloride. => Chapter6 9
Chapter 6 9 “Trivial” Names • CH2X2 called methylene halide. • CHX3 is a haloform. • CX4 is carbon tetrahalide. • Examples: ➢CH2Cl2 is methylene chloride ➢CHCl3 is chloroform ➢CCl4 is carbon tetrachloride. =>

Uses of Alkyl Halides Solvents -degreasers and dry cleaning fluid Reagents for synthesis of other compounds Anesthetic:Halothane is CF3CHCIBr >CHCI3 used originally (toxic and carcinogenic) Freons,chlorofluorocarbons or CFC's >Freon 12,CF2Cl2,now replaced with Freon 22, CF2CHCI,not as harmful to ozone layer. Pesticides -DDT banned in U.S. => Chapter6 10
Chapter 6 10 Uses of Alkyl Halides • Solvents - degreasers and dry cleaning fluid • Reagents for synthesis of other compounds • Anesthetic: Halothane is CF3CHClBr ➢CHCl3 used originally (toxic and carcinogenic) • Freons, chlorofluorocarbons or CFC’s ➢Freon 12, CF2Cl2 , now replaced with Freon 22, CF2CHCl, not as harmful to ozone layer. • Pesticides - DDT banned in U.S. =>
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 03 Structure and Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 01 Introduction and Review(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 06 Haluros de Alquilo - Substitución Nucleofílica y Eliminación.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
