《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 05 Stereochemistry

Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 5 Stereochemistry Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
Chapter 5 Stereochemistry Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr

Chirality ·“Handedness"”:right glove doesn't fit the left hand. Mirror-image object is different from the original object. =>
Chapter 5 2 Chirality • “Handedness”: right glove doesn’t fit the left hand. • Mirror-image object is different from the original object. =>
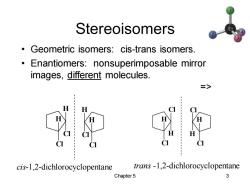
Stereoisomers Geometric isomers:cis-trans isomers. Enantiomers:nonsuperimposable mirror images,different molecules. => cis-1,2-dichlorocyclopentane trans-1,2-dichlorocyclopentane Chapter 5 3
Chapter 5 3 Stereoisomers • Geometric isomers: cis-trans isomers. • Enantiomers: nonsuperimposable mirror images, different molecules. => cis-1,2-dichlorocyclopentane trans -1,2-dichlorocyclopentane H Cl H Cl H Cl H Cl H Cl Cl H H Cl Cl H
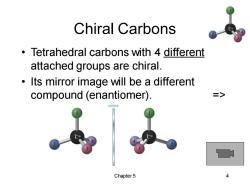
Chiral Carbons Tetrahedral carbons with 4 different attached groups are chiral. Its mirror image will be a different compound (enantiomer). > Chapter5
Chapter 5 4 Chiral Carbons • Tetrahedral carbons with 4 different attached groups are chiral. • Its mirror image will be a different compound (enantiomer). =>
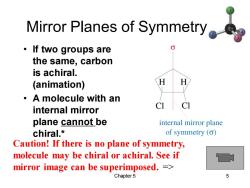
Mirror Planes of Symmetry ·If two groups are the same,carbon is achiral. (animation) 。A molecule with an C 1 internal mirror plane cannot be internal mirror plane chiral.* of symmetry (o) Caution!If there is no plane of symmetry, molecule may be chiral or achiral.See if mirror image can be superimposed.= Chapter 5 5
Chapter 5 5 Mirror Planes of Symmetry • If two groups are the same, carbon is achiral. (animation) • A molecule with an internal mirror plane cannot be chiral.* Caution! If there is no plane of symmetry, molecule may be chiral or achiral. See if mirror image can be superimposed. =>
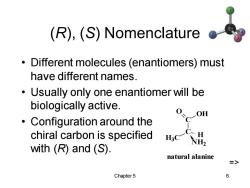
(R),(S)Nomenclature Different molecules (enantiomers)must have different names. Usually only one enantiomer will be biologically active. Configuration around the C-OH chiral carbon is specified C…H NH2 with (R)and (S). natural alanine > Chapter 5 6
Chapter 5 6 (R), (S) Nomenclature • Different molecules (enantiomers) must have different names. • Usually only one enantiomer will be biologically active. • Configuration around the chiral carbon is specified with (R) and (S). C C O OH H3C NH2 H natural alanine =>

Cahn-Ingold-Prelog Rules Assign a priority number to each group attached to the chiral carbon. Atom with highest atomic number assigned the highest priority #1. In case of ties,look at the next atoms along the chain. Double and triple bonds are treated like bonds to duplicate atoms. => Chapter 5
Chapter 5 7 Cahn-Ingold-Prelog Rules • Assign a priority number to each group attached to the chiral carbon. • Atom with highest atomic number assigned the highest priority #1. • In case of ties, look at the next atoms along the chain. • Double and triple bonds are treated like bonds to duplicate atoms. =>
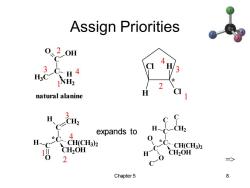
Assign Priorities 0、20H C 3C..H4 H3C NH2 natural alanine H expands to H 4 H *C CH(CH3)2 CH(CH3)2 CH2OH H CH2OH 2 c-0 Chapter 5 8
Chapter 5 8 Assign Priorities C C O OH H3C NH2 H natural alanine 1 2 3 4 Cl Cl H H * 1 2 3 4 1 2 3 4 => C C O H C H CH2 CH2OH CH(CH3) 2 * C C C CH2OH CH(CH3 ) 2 H O O C C H CH2 C * expands to
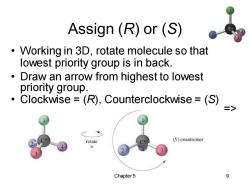
Assign(R)or(S) Working in 3D,rotate molecule so that 。 lowest priority group is in back. Draw an arrow from highest to lowest priority group. Clockwise =(R),Counterclockwise =(S) rotate (S)enantiomer Chapter 5 9
Chapter 5 9 Assign (R) or (S) • Working in 3D, rotate molecule so that lowest priority group is in back. • Draw an arrow from highest to lowest priority group. • Clockwise = (R), Counterclockwise = (S) =>
Properties of Enantiomers Same boiling point,melting point,density Same refractive index Different direction of rotation in polarimeter Different interaction with other chiral molecules Enzymes Taste buds,scent => Chapter 5 10
Chapter 5 10 Properties of Enantiomers • Same boiling point, melting point, density • Same refractive index • Different direction of rotation in polarimeter • Different interaction with other chiral molecules – Enzymes – Taste buds, scent =>
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 03 Structure and Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 01 Introduction and Review(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 06 Haluros de Alquilo - Substitución Nucleofílica y Eliminación.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 25 Lipids.ppt
