《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 20 Carboxylic Acids

Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 20 Carboxylic Acids Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
Chapter 20 Carboxylic Acids Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr

Introduction The functional group of carboxylic acids consists of a C=O with -OH bonded to the same carbon. Carboxyl group is usually written -COOH. Aliphatic acids have an alkyl group bonded to -COOH. Aromatic acids have an aryl group. Fatty acids are long-chain aliphatic acids. > Chapter 20
Chapter 20 2 Introduction • The functional group of carboxylic acids consists of a C=O with -OH bonded to the same carbon. • Carboxyl group is usually written -COOH. • Aliphatic acids have an alkyl group bonded to -COOH. • Aromatic acids have an aryl group. • Fatty acids are long-chain aliphatic acids. =>
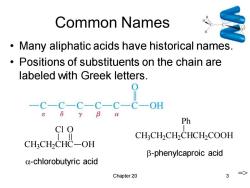
Common Names Many aliphatic acids have historical names. Positions of substituents on the chain are labeled with Greek letters. -C-C-C-C一C-C OH 8 Ph CI O CH:CH2CH2CHCH2COOH CH3CH2CHC-OH β-phenylcaproic acid a-chlorobutyric acid Chapter 20 3 =>
Chapter 20 3 Common Names • Many aliphatic acids have historical names. • Positions of substituents on the chain are labeled with Greek letters. CH3 CH2 CHC Cl OH O -chlorobutyric acid CH3 CH2 CH2 CHCH2 COOH Ph -phenylcaproic acid =>

IUPAC Names Remove -e from alkane(or alkene) name,add -oic acid. The carbon of the carboxyl group is #1. CIO H CH:CH-CHC-OH Ph-c=C H COOH 2-chlorobutanoic acid trans-3-phenyl-2-propenoic acid (cinnamic acid) => Chapter 20
Chapter 20 4 IUPAC Names • Remove -e from alkane (or alkene) name, add -oic acid. • The carbon of the carboxyl group is #1. CH3 CH2 CHC Cl OH O 2-chlorobutanoic acid Ph C H C H COOH trans-3-phenyl-2-propenoic acid (cinnamic acid) =>
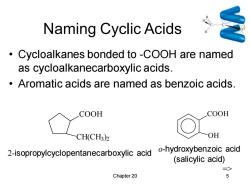
Naming Cyclic Acids Cycloalkanes bonded to -COOH are named as cycloalkanecarboxylic acids. Aromatic acids are named as benzoic acids. COOH COOH CH(CH3)2 OH 2-isopropylcyclopentanecarboxylic acid -hydroxybenzoic acid (salicylic acid) => Chapter 20 5
Chapter 20 5 Naming Cyclic Acids • Cycloalkanes bonded to -COOH are named as cycloalkanecarboxylic acids. • Aromatic acids are named as benzoic acids. COOH CH(CH3 ) 2 2-isopropylcyclopentanecarboxylic acid COOH OH o-hydroxybenzoic acid (salicylic acid) =>

Dicarboxylic Acids Aliphatic diacids are usually called by their common names (to be memorized). For IUPAC name,number the chain from the end closest to a substituent. Two carboxyl groups on a benzene ring indicate a phthalic acid. Br HOOCCH2CHCH2CH2COOH 3-bromohexanedioic acid B-bromoadipic acid Chapter 20 6
Chapter 20 6 Dicarboxylic Acids • Aliphatic diacids are usually called by their common names (to be memorized). • For IUPAC name, number the chain from the end closest to a substituent. • Two carboxyl groups on a benzene ring indicate a phthalic acid. HOOCCH2 CHCH2 CH2 COOH Br 3-bromohexanedioic acid -bromoadipic acid =>
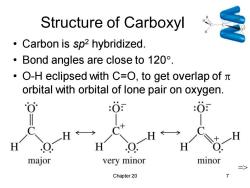
Structure of Carboxyl Carbon is sp2 hybridized. ·Bond angles are close to120°. ·O-H eclipsed with C=O,to get overlap ofπ orbital with orbital of lone pair on oxygen. :O major very minor minor Chapter 20
Chapter 20 7 Structure of Carboxyl • Carbon is sp2 hybridized. • Bond angles are close to 120. • O-H eclipsed with C=O, to get overlap of orbital with orbital of lone pair on oxygen. =>
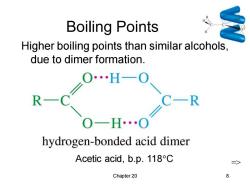
Boiling Points Higher boiling points than similar alcohols, due to dimer formation. ∠0H-0 R C- R 0-H…0 hydrogen-bonded acid dimer Acetic acid,b.p.118C 二> Chapter 20 8
Chapter 20 8 Boiling Points Higher boiling points than similar alcohols, due to dimer formation. Acetic acid, b.p. 118C =>

Melting Points Aliphatic acids with more than 8 carbons are solids at room temperature. Double bonds (especially cis)lower the melting point.Note these 18-C acids: >Stearic acid (saturated):72C >Oleic acid (one cis double bond):16C >Linoleic acid (two cis double bonds):-5C => Chapter 20 9
Chapter 20 9 Melting Points • Aliphatic acids with more than 8 carbons are solids at room temperature. • Double bonds (especially cis) lower the melting point. Note these 18-C acids: ➢Stearic acid (saturated): 72C ➢Oleic acid (one cis double bond): 16C ➢Linoleic acid (two cis double bonds): -5C =>

Solubility Water solubility decreases with the length of the carbon chain. Up to 4 carbons,acid is miscible in water. More soluble in alcohol. Also soluble in relatively nonpolar solvents like chloroform because it dissolves as a dimer. => Chapter 20 10
Chapter 20 10 Solubility • Water solubility decreases with the length of the carbon chain. • Up to 4 carbons, acid is miscible in water. • More soluble in alcohol. • Also soluble in relatively nonpolar solvents like chloroform because it dissolves as a dimer. =>
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 03 Structure and Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, 6th Edition L. G. Wade, Jr.)Chapter 01 Introduction and Review(西北农林科技大学,2010).ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 06 Haluros de Alquilo - Substitución Nucleofílica y Eliminación.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
