《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions

Organic Chemistry,7th Edition H. L.G.Wade,Jr. H Chapter 4 The Study of Chemical Reactions Copyright 2010 Pearson Education,Inc
Chapter 4 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. The Study of Chemical Reactions

Introduction ·Overall reaction:reactants→products Mechanism:Step-by-step pathway. To learn more about a reaction: ·Thermodynamics Kinetics Chapter 4 2
Chapter 4 2 Introduction • Overall reaction: reactants → products • Mechanism: Step-by-step pathway. • To learn more about a reaction: ▪ Thermodynamics ▪ Kinetics
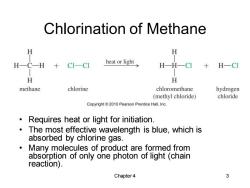
Chlorination of Methane H H H一CH CI-CI heat or light H-H-CI H-CI H H methane chlorine chloromethane hydrogen (methyl chloride) chloride Copyright2010 Pearson Prentice Hall,Inc. Requires heat or light for initiation. The most effective wavelength is blue,which is absorbed by chlorine gas. Many molecules of product are formed from absorption of only one photon of light (chain reaction). Chapter 4 3
Chapter 4 3 Chlorination of Methane • Requires heat or light for initiation. • The most effective wavelength is blue, which is absorbed by chlorine gas. • Many molecules of product are formed from absorption of only one photon of light (chain reaction)

The Free-Radical Chain Reaction Initiation generates a radical intermediate. Propagation:The intermediate reacts with a stable molecule to produce another reactive intermediate(and a product molecule). Termination:Side reactions that destroy the reactive intermediate. Chapter 4 4
Chapter 4 4 The Free-Radical Chain Reaction • Initiation generates a radical intermediate. • Propagation: The intermediate reacts with a stable molecule to produce another reactive intermediate (and a product molecule). • Termination: Side reactions that destroy the reactive intermediate
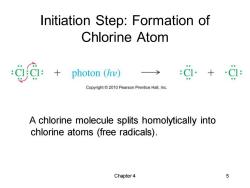
Initiation Step:Formation of Chlorine Atom photon (hv) :g+ Copyright2010 Pearson Prentice Hall,Inc. A chlorine molecule splits homolytically into chlorine atoms (free radicals). Chapter4 5
Chapter 4 5 Initiation Step: Formation of Chlorine Atom A chlorine molecule splits homolytically into chlorine atoms (free radicals)
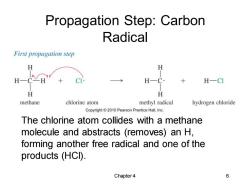
Propagation Step:Carbon Radical First propagation step H CH H-CI H H methane chlorine atom methyl radical hydrogen chloride Copyright2010 Pearson Prentice Hall,Inc. The chlorine atom collides with a methane molecule and abstracts (removes)an H, forming another free radical and one of the products (HCI). Chapter 4 6
Chapter 4 6 Propagation Step: Carbon Radical The chlorine atom collides with a methane molecule and abstracts (removes) an H, forming another free radical and one of the products (HCl)
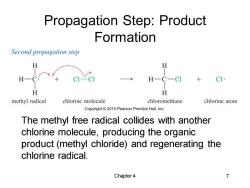
Propagation Step:Product Formation Second propagation step H H H- C-CI H H methyl radical chlorine molecule chloromethane chlorine atom Copyright2010 Pearson Prentice Hall,Inc. The methyl free radical collides with another chlorine molecule,producing the organic product(methyl chloride)and regenerating the chlorine radical. Chapter 4 7
Chapter 4 7 Propagation Step: Product Formation The methyl free radical collides with another chlorine molecule, producing the organic product (methyl chloride) and regenerating the chlorine radical

Overall Reaction photon ( :C+C1: First propagation step H H H- H H一C H一CI H H methane chlorine atom methyl radical hydrogen chloride Second propagation step H H H-C. H一C-C CI methyl radical chlorine molecule chloromethane chlorine atom H H HCH+C一CI heat or light H-H-CI+H一C H H methane chlorine chloromethane hydrogen (methyl chloride) chloride Chapter 4 8
Chapter 4 8 Overall Reaction
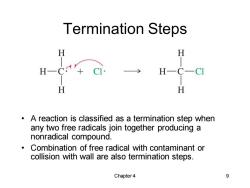
Termination Steps H H H H H A reaction is classified as a termination step when any two free radicals join together producing a nonradical compound. Combination of free radical with contaminant or collision with wall are also termination steps. Chapter4 9
Chapter 4 9 Termination Steps • A reaction is classified as a termination step when any two free radicals join together producing a nonradical compound. • Combination of free radical with contaminant or collision with wall are also termination steps
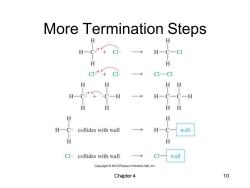
More Termination Steps H一( C H CI-CI H HH ·CH H一C一C一H H H HH H H H collides with wall wall H H Cl·collides with wall wall Copyright 2010 Pearson Prentice Hall,Inc Chapter 4 10
Chapter 4 10 More Termination Steps
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 21 Part 1 - Structure and Properties of Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 22 Condensations and Alpha Substitutions of Carbonyl Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
