《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 08 Reactions of Alkenes

Organic Chemistry,7th Edition L.G.Wade,Jr. Chapter 8 Reactions of Alkenes Copyright 2010 Pearson Education,Inc
Chapter 8 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Reactions of Alkenes
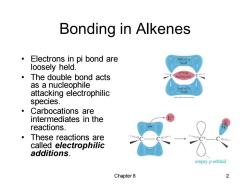
Bonding in Alkenes Electrons in pi bond are half of n loosely held. ·The double bond acts as a nucleophile hof元 attacking electrophilic hond species. ·Carbocations are intermediates in the reactions. ·These reactions are called electrophilic additions. empty p orbital Chapter 8 2
Chapter 8 2 Bonding in Alkenes • Electrons in pi bond are loosely held. • The double bond acts as a nucleophile attacking electrophilic species. • Carbocations are intermediates in the reactions. • These reactions are called electrophilic additions

Electrophilic Addition Step 1:Pi electrons attack the electrophile. E on the more substituted carbon Step 2:Nucleophile attacks the carbocation. +Nuc:- E Nuc Copyright 2010 Pearson Prentice Hall.Inc. Chapter 8 3
Chapter 8 3 Electrophilic Addition • Step 1: Pi electrons attack the electrophile. • Step 2: Nucleophile attacks the carbocation

Types of Additions TABLE 8-1 Types of Additions to Alkenes C=C Type of Addition [Elements Added]a Product hydration halogenation [H.O [X.).an oxidation hydrogenation halohydrin formation [H.I.a reduction [HOX].an oxidation hydroxylation HX addition [HOOH],an oxidation HX灯 (hydrohalogenation) oxidative cleavage C=00=( [O.I.an oxidation cyclopropanation CH】 O].an oxidation These are not the reagents used but simply the groups that appear in the produc Copyright2010 Pearson Prentice Hall,Inc. Chapter8 4
Chapter 8 4 Types of Additions

Addition of HX to Alkenes Step 1 is the protonation of the double bond. The protonation step forms the most stable carbocation possible. In step 2,the nucleophile attacks the carbocation,forming an alkyl halide. HBr,HCI,and HI can be added through this reaction. Chapter8 5
Chapter 8 5 Addition of HX to Alkenes • Step 1 is the protonation of the double bond. • The protonation step forms the most stable carbocation possible. • In step 2, the nucleophile attacks the carbocation, forming an alkyl halide. • HBr, HCl, and HI can be added through this reaction
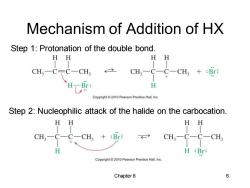
Mechanism of Addition of HX Step 1:Protonation of the double bond. HH HH CH3一CC一CH3 CH3-C-C-CH3+:Br: H-Br: H Copyright 2010 Pearson Prentice Hall.Inc. Step 2:Nucleophilic attack of the halide on the carbocation. HH HH CH3一 C一CH3+:Br CH3-C- CH H H :Br: Copyright2010 Pearson Prentice Hall,Inc Chapter 8 6
Chapter 8 6 Mechanism of Addition of HX Step 1: Protonation of the double bond. Step 2: Nucleophilic attack of the halide on the carbocation

Regioselectivity Markovnikov's Rule:The addition of a proton to the double bond of an alkene results in a product with the acidic proton bonded to the carbon atom that already holds the greater number of hydrogens. Markovnikov's Rule (extended):In an electrophilic addition to the alkene,the electrophile adds in such a way that it generates the most stable intermediate Chapter 8 7
Chapter 8 7 Regioselectivity • Markovnikov’s Rule: The addition of a proton to the double bond of an alkene results in a product with the acidic proton bonded to the carbon atom that already holds the greater number of hydrogens. • Markovnikov’s Rule (extended): In an electrophilic addition to the alkene, the electrophile adds in such a way that it generates the most stable intermediate

Markovnikov's Rule CH; CH CH3一CFCH-CH add H+to secondary carbon CH;-C-CH-CH3 HBr H Br tertiary carbocation CH, CH CH3一CCH一CH add H to tertiary carbon CH,一C-CH-CH H Br secondary carbocation Copyright2010 Pearson Prentice Hall,Inc. The acid proton will bond to carbon 3 in order to produce the most stable carbocation possible. Chapter8 8
Chapter 8 8 Markovnikov’s Rule The acid proton will bond to carbon 3 in order to produce the most stable carbocation possible

Free-Radical Addition of HBr In the presence of peroxides,HBr adds to an alkene to form the "anti-Markovnikov'product. Peroxides produce free radicals. Only HBr has the right bond energy. The HCI bond is too strong,so it will add according to Markovnikov's rule,even in the presence of peroxide. The HI bond tends to break heterolytically to form ions,it too will add according to Markovnikov's rule. Chapter 8 9
Chapter 8 9 Free-Radical Addition of HBr • In the presence of peroxides, HBr adds to an alkene to form the “anti-Markovnikov” product. • Peroxides produce free radicals. • Only HBr has the right bond energy. • The HCl bond is too strong, so it will add according to Markovnikov’s rule, even in the presence of peroxide. • The HI bond tends to break heterolytically to form ions, it too will add according to Markovnikov’s rule
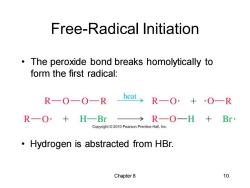
Free-Radical Initiation The peroxide bond breaks homolytically to form the first radical: R-O—O—R heat> R一O·+·O一R R一O·+H一Br >R—O—H+Br Copyright 2010 Pearson Prentice Hall,Inc. Hydrogen is abstracted from HBr. Chapter8 10
Chapter 8 10 Free-Radical Initiation • The peroxide bond breaks homolytically to form the first radical: • Hydrogen is abstracted from HBr
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 21 Part 1 - Structure and Properties of Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 22 Condensations and Alpha Substitutions of Carbonyl Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules.ppt
