《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules
Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 2 Structure and Properties of Organic Molecules Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 2 Structure and Properties of Organic Molecules Organic Chemistry, 6th Edition L. G. Wade, Jr. Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall
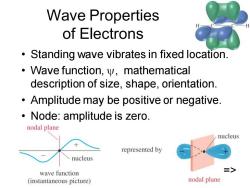
Wave Properties of Electrons Standing wave vibrates in fixed location. Wave function,y,mathematical description of size,shape,orientation. Amplitude may be positive or negative. Node:amplitude is zero. nodal plane nucleus represented by nucleus wave function > (instantaneous picture) nodal plane
Chapter 2 2 Wave Properties of Electrons • Standing wave vibrates in fixed location. • Wave function, , mathematical description of size, shape, orientation. • Amplitude may be positive or negative. • Node: amplitude is zero. =>
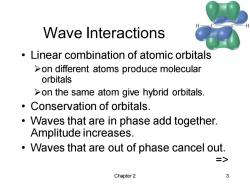
Wave Interactions Linear combination of atomic orbitals >on different atoms produce molecular orbitals >on the same atom give hybrid orbitals. Conservation of orbitals. Waves that are in phase add together. Amplitude increases. Waves that are out of phase cancel out. Chapter 2 3
Chapter 2 3 Wave Interactions • Linear combination of atomic orbitals ➢on different atoms produce molecular orbitals ➢on the same atom give hybrid orbitals. • Conservation of orbitals. • Waves that are in phase add together. Amplitude increases. • Waves that are out of phase cancel out. =>
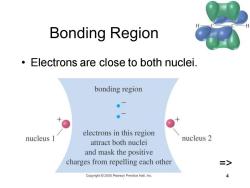
Bonding Region Electrons are close to both nuclei. bonding region ●入 electrons in this region nucleus 1 attract both nuclei nucleus 2 and mask the positive charges from repelling each other > Copyright2005 Pearson Prentice Hall.Inc
Chapter 2 4 Bonding Region • Electrons are close to both nuclei. =>

Sigma Bonding Electron density lies between the nuclei. A bond may be formed by s-s,p-p,s-p, or hybridized orbital overlaps. The bonding MO is lower in energy than the original atomic orbitals. The antibonding MO is higher in energy than the atomic orbitals. => Chapter 2 5
Chapter 2 5 Sigma Bonding • Electron density lies between the nuclei. • A bond may be formed by s-s, p-p, s-p, or hybridized orbital overlaps. • The bonding MO is lower in energy than the original atomic orbitals. • The antibonding MO is higher in energy than the atomic orbitals. =>
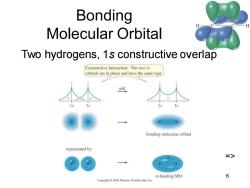
Bonding Molecular Orbital Two hydrogens,1s constructive overlap Constructive Interaction:The two Is orbitals are in phase and have the same sign add bonding molecular orbital represented by: => σ-bonding MO 6 Copyright2005 Pearson Prentice Hall,Inc
Chapter 2 6 Bonding Molecular Orbital Two hydrogens, 1s constructive overlap =>
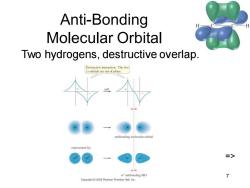
Anti-Bonding Molecular Orbital Two hydrogens,destructive overlap. Destructive interaction:The twe 1s orbitals are out of phase. anubonding molecular orbital represented by: => node antibonding MO 7 Pearson Prentice Hall,Inc
Chapter 2 7 Anti-Bonding Molecular Orbital Two hydrogens, destructive overlap. =>
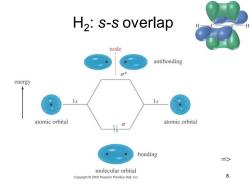
H2:s-s overlap node antibonding 0* energy atomic orbital atomic orbital bonding 三> molecular orbital Copyright 2005 Pearson Prentice Hall,Inc. 8
Chapter 2 8 H2 : s-s overlap =>
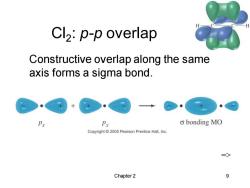
Cl2:p-p overlap Constructive overlap along the same axis forms a sigma bond. Px Px o bonding MO Copyright 2005 Pearson Prentice Hall,Inc. => Chapter 2 9
Chapter 2 9 Cl2 : p-p overlap => Constructive overlap along the same axis forms a sigma bond
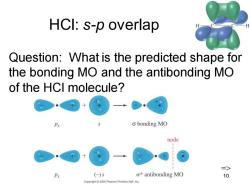
HCI:s-p overlap Question:What is the predicted shape for the bonding MO and the antibonding MO of the HCI molecule? o bonding MO node => Px (-)s o*antibonding MO 10 Copyright 2005 Pearson Prentice Hall.Inc
Chapter 2 10 HCl: s-p overlap Question: What is the predicted shape for the bonding MO and the antibonding MO of the HCl molecule? =>
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 10 Alkyl Halides.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 09 Stereochemistry.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 08 Alkynes.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 07 Alkenes - Reactions and Synthesis.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 06 Alkenes - Structure and Reactivity.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 05 An Overview of Organic Reactions.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 04 Stereochemistry of Alkanes and Cycloalkanes.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 03 Organic Compounds - Alkanes and Cycloalkanes.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 02 Polar Covalent Bonds; Acids and Bases.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 01 Structure and Bonding.pdf
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2008—2009学年第二学期ORGANIC CHEMISTRY课程A卷(答案).doc
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2008—2009学年第二学期ORGANIC CHEMISTRY课程A卷(试题).doc
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2012—2013学年第2学期课程A卷(答案).pdf
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2012—2013学年第2学期课程A卷(试题).pdf
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2004年有机化学期末测试题B(双语)试题.pdf
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2004年有机化学期末测试题B(双语)答案.pdf
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 08 Alkenes - Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 21 Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 23 Carbohydrates and Nucleic Acids.ppt
