《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 08 Alkynes
Alkynes Based on McMurry's Organic Chemistry,6th edition,Chapter 8
Alkynes Based on McMurry’s Organic Chemistry, 6th edition, Chapter 8
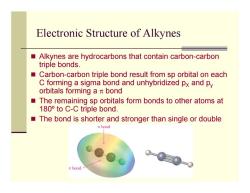
Electronic Structure of Alkynes Alkynes are hydrocarbons that contain carbon-carbon triple bonds. ■ Carbon-carbon triple bond result from sp orbital on each C forming a sigma bond and unhybridized px and py orbitals forming a t bond The remaining sp orbitals form bonds to other atoms at 180 to C-C triple bond. The bond is shorter and stronger than single or double T bond m bond
Electronic Structure of Alkynes Alkynes are hydrocarbons that contain carbon-carbon triple bonds. Carbon-carbon triple bond result from sp orbital on each C forming a sigma bond and unhybridized p X and p y orbitals forming a π bond The remaining sp orbitals form bonds to other atoms at 180º to C-C triple bond. The bond is shorter and stronger than single or double

Naming Alkynes ■ General hydrocarbon rules apply with "-yne"as a suffix indicating an alkyne ■ Numbering of chain with triple bond is set so that the smallest number possible include the triple bond 8 7 65 432 CH CHCHCH2C=CCH2CH Begin numbering at the end nearer the triple bond. CHg 6-Methyl-3-octyne A compound with two triple bonds is a diyne A triyne has three triple bonds An enyne has a double bond and triple bond
Naming Alkynes General hydrocarbon rules apply with “-yne” as a suffix indicating an alkyne Numbering of chain with triple bond is set so that the smallest number possible include the triple bond A compound with two triple bonds is a diyne A triyne has three triple bonds An enyne has a double bond and triple bond
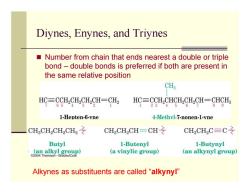
Diynes,Enynes,and Triynes Number from chain that ends nearest a double or triple bond-double bonds is preferred if both are present in the same relative position CH3 HC三CCH2CHCH2CH=CH2 HC=CCH,CHCH2CH2CH=CHCH3 65 3 2 1 23 45 89 1-Hepten-6-vne 4-Methvl-7-nonen-1-vne CHCH2CH2CH2之 CH CH2CH-CH CH3CH2C=C之 Butyl 1-Butenyl 1-Butynyl 4aal黑e (a vinylic group) (an alkynyl group) Alkynes as substituents are called "alkynyl
Diynes, Enynes, and Triynes Number from chain that ends nearest a double or triple bond – double bonds is preferred if both are present in the same relative position Alkynes as substituents are called “alkynyl
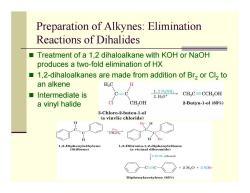
Preparation of Alkynes:Elimination Reactions of Dihalides ■ Treatment of a 1,2 dihaloalkane with KOH or NaOH produces a two-fold elimination of HX ■ 1,2-dihaloalkanes are made from addition of Br2 or Cl2 to an alkene HC ■Intermediate is 1.2 NaNH2 2.Hg0+ CHC=CCH,OH a vinyl halide C CH2OH 2-Butyn-1-ol (85%) 3-Chloro-2-buten-1-ol (a vinvlic chloride) Br H B型 1,2-Diphenylethylene (Stilbene) 1-Dticina)thane 2 KOH,ethanol C=C- +2H2O+2B Diphenylacetylene(85%)
Preparation of Alkynes: Elimination Reactions of Dihalides Treatment of a 1,2 dihaloalkane with KOH or NaOH produces a two-fold elimination of HX 1,2-dihaloalkanes are made from addition of Br 2 or Cl 2 to an alkene Intermediate is a vinyl halide

Reactions of Alkynes:Addition of HX and X2 Addition reactions of alkynes are similar to those of alkenes Intermediate alkene reacts further with excess reagent Regiospecificity according to Markovnikov's Rule Br H Br H CH CHCH2CH2C=CH HBr CH2CO2H CH CH2CH2CH,C=CH HBr CH CH2CH2CH2C-C-H 1-Hexyne 2-Bromo-1-hexene Br H 2,2-Dibromohexane CH,CH CH CH2C=CCH2CH HCI.NH CI C=C CH3CO2H 3-Hexyne CH,CH2 H (Z)-3-Chloro-3-hexene (95%)
Reactions of Alkynes: Addition of HX and X 2 Addition reactions of alkynes are similar to those of alkenes Intermediate alkene reacts further with excess reagent Regiospecificity according to Markovnikov’s Rule
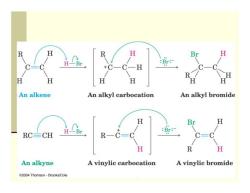
R R H Br H-Br :Br:- +0 H H H An alkene An alkyl carbocation An alkyl bromide H Br H :Br: RC=CH R一 H R H An alkyne A vinylic carbocation A vinylic bromide 2004 Thomson-Brooks/Cole
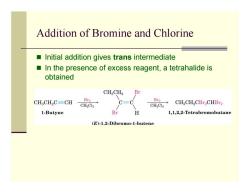
Addition of Bromine and Chlorine ■ Initial addition gives trans intermediate In the presence of excess reagent,a tetrahalide is obtained CHCH2 Br CH CH2C=CH Br2 Br2 CH2Cl2 CH2Cl2 CHCH2CBr2CHBr2 1-Butyne Br H 1,1,2,2-Tetrabromobutane (E)-1.2-Dibromo-1-butene
Addition of Bromine and Chlorine Initial addition gives trans intermediate In the presence of excess reagent, a tetrahalide is obtained
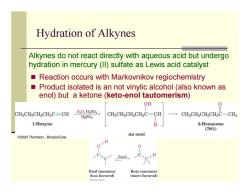
Hydration of Alkynes Alkynes do not react directly with aqueous acid but undergo hydration in mercury(Il)sulfate as Lewis acid catalyst Reaction occurs with Markovnikov regiochemistry Product isolated is an not vinylic alcohol (also known as enol)but a ketone(keto-enol tautomerism) OH CHCH2CH2CH2C=CH H2O,H2SO4 CHCH2CH2CH2C-CH HgsO4 CHgCH2CH2CH2C-CHg 1-Hexyne H 2-Hexanone (786) An enol C2004 Thomson-Brooks/Cole H 0 Rapid H Enol tautomer Keto tautomer (Icss favorcd) (more favorcd) Toe-Dhoek
Reaction occurs with Markovnikov regiochemistry Product isolated is an not vinylic alcohol (also known as enol) but a ketone (keto-enol tautomerism) Hydration of Alkynes Alkynes do not react directly with aqueous acid but undergo hydration in mercury (II) sulfate as Lewis acid catalyst

Mechanism of Mercury(ID)-Catalyzed Hydration of Alkynes The alkyne uses a pair of electrons to R一C attack the electrophilic mercury(I) Hg2+802 ion,yielding a mercury-containing ■ Electrophilic vinylie carbocation intermediate. addition of Hg(Il)to Nucleophilic attack of water on Hg'SO alkyne gives a the earbocation forms a C-O bond and yields a protonated mercury- vinylic cation containing enol. ■Vater adds and Hg+802 loses a proton ■Acidic reaction +HO condition is e olacement of Hg'by H'occurs sufficient to replace to give a neutral enol mercury by H +HO The enol under to give e the final ketone product
Mechanism of Mercury(II)-Catalyzed Hydration of Alkynes Electrophilic addition of Hg(II) to alkyne gives a vinylic cation Water adds and loses a proton Acidic reaction condition is sufficient to replace mercury by H
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 07 Alkenes - Reactions and Synthesis.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 06 Alkenes - Structure and Reactivity.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 05 An Overview of Organic Reactions.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 04 Stereochemistry of Alkanes and Cycloalkanes.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 03 Organic Compounds - Alkanes and Cycloalkanes.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 02 Polar Covalent Bonds; Acids and Bases.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 01 Structure and Bonding.pdf
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2008—2009学年第二学期ORGANIC CHEMISTRY课程A卷(答案).doc
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2008—2009学年第二学期ORGANIC CHEMISTRY课程A卷(试题).doc
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2012—2013学年第2学期课程A卷(答案).pdf
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2012—2013学年第2学期课程A卷(试题).pdf
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2004年有机化学期末测试题B(双语)试题.pdf
- 西北农林科技大学:《有机化学》课程教学资源(试卷习题)2004年有机化学期末测试题B(双语)答案.pdf
- 《有机化学》课程教学资源(参考书籍)Solution manual Organic Chemistry(SIXTH EDITION, 2005, L. G. Wade, Jr., Jan William Simek).pdf
- 《有机化学》课程教学资源(参考书籍)Organic Chemistry, 8th Edition, L . G . WA D E , J R ..pdf
- 《有机化学》课程教学资源(参考书籍)Organic Chemistry(7th Edition)Prentice Hall(2009, L.G. Wade, Jr. Whitman College).pdf
- 《有机化学》课程教学资源(参考书籍)Solutions Manual for Organic Chemistry-Prentice Hall(8th Edition, 2012, L.G.WADE,JR., Jan William Simek).pdf
- 《有机化学》课程教学资源(参考书籍)Organic Chemistry With Biological Applications, 2nd Edition -Brooks Cole(2010, John McMurry, Cornell University).pdf
- 《有机化学》课程教学资源(参考书籍)Organic chemistry-Brooks(7th edition, 2014, William H. Brown Brent L. Iverson Eric V. Anslyn Christopher S. Foote Bruce M. Novak).pdf
- 《有机化学》课程教学资源(参考书籍)Keynotes in Organic Chemistry, Second Edition, Andrew F. Parsons(Wiley, 2014).pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 09 Stereochemistry.pdf
- 《有机化学》课程教学课件(McMurry’s Organic Chemistry, 6th edition)Chapter 10 Alkyl Halides.pdf
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry,5th Edition,L. G. Wade, Jr.,Prentice Hall)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 08 Alkenes - Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 09 Alkynes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 10 Structure and Synthesis of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry 6th LG Wade by Prentice Hall,L. G. Wade, Jr.)Chapter 16 Aromatic Compounds.ppt
