《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination

Organic Chemistry, ORGANIC 8th Edition CHEMISTRY L.G.Wade,Jr. Chapter 6 Lecture Alkyl Halides: Nucleophilic Substitution and Elimination G.WADE,J R Rizalia Klausmeyer Baylor University Waco,TX 2013 Pearson Education,Inc ALWAYS LEARNING PEARSON
Chapter 6 Lecture Organic Chemistry, 8 th Edition L. G. Wade, Jr. Alkyl Halides: Nucleophilic Substitution and Elimination © 2013 Pearson Education, Inc. Rizalia Klausmeyer Baylor University Waco, TX
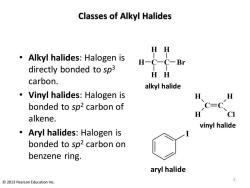
Classes of Alkyl Halides HH Alkyl halides:Halogen is H-C-C-Br directly bonded to sp3 HH carbon. alkyl halide Vinyl halides:Halogen is 及、 H bonded to sp2 carbon of C=C H CI alkene. vinyl halide Aryl halides:Halogen is bonded to sp2 carbon on benzene ring. aryl halide 2013 Pearson Education Inc
Classes of Alkyl Halides • Alkyl halides: Halogen is directly bonded to sp3 carbon. • Vinyl halides: Halogen is bonded to sp2 carbon of alkene. • Aryl halides: Halogen is bonded to sp2 carbon on benzene ring. C C H H H Cl vinyl halide C H H H C H H Br alkyl halide I aryl halide 2 © 2013 Pearson Education Inc
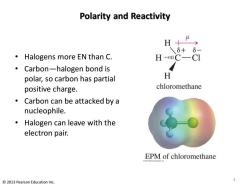
Polarity and Reactivity H+ 6+ 6 Halogens more EN than C. HC- CI Carbon-halogen bond is polar,so carbon has partial H positive charge. chloromethane Carbon can be attacked by a nucleophile. Halogen can leave with the electron pair. EPM of chloromethane 2013 Pearson Education Inc
Polarity and Reactivity • Halogens more EN than C. • Carbon—halogen bond is polar, so carbon has partial positive charge. • Carbon can be attacked by a nucleophile. • Halogen can leave with the electron pair. 3 © 2013 Pearson Education Inc
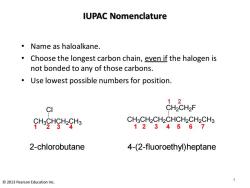
IUPAC Nomenclature ·Name as haloalkane. Choose the longest carbon chain,even if the halogen is not bonded to any of those carbons. Use lowest possible numbers for position. 12 CI CH2CH2F CHoCHgHgHa CH3CH2CH2CHCH2CH2CH3 1234567 2-chlorobutane 4-(2-fluoroethyl)heptane 2013 Pearson Education Inc
IUPAC Nomenclature • Name as haloalkane. • Choose the longest carbon chain, even if the halogen is not bonded to any of those carbons. • Use lowest possible numbers for position. CH3CH2CH2CHCH2CH2CH3 CH2CH2 F 1 2 3 4 2-chlorobutane 4-(2-fluoroethyl)heptane 1 2 3 4 5 6 7 1 2 CH3CHCH2CH3 Cl 4 © 2013 Pearson Education Inc
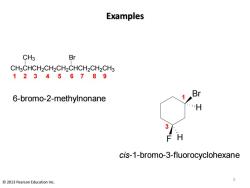
Examples CH3 Br CH3CHCH2CH2CH2CHCH2CH2CH3 123456789 Br 6-bromo-2-methylnonane H 3 FH cis-1-bromo-3-fluorocyclohexane 2013 Pearson Education Inc
Examples CH3CHCH2CH2CH2CHCH2CH2CH3 CH3 Br Br F H H 1 2 3 4 5 6 7 8 9 6-bromo-2-methylnonane 1 3 cis-1-bromo-3-fluorocyclohexane 5 © 2013 Pearson Education Inc
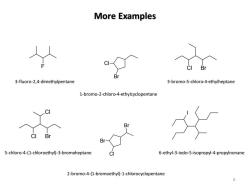
More Examples Br 3-fluoro-2,4-dimethylpentane 3-bromo-5-chloro-4-ethylheptane 1-bromo-2-chloro-4-ethylcyclopentane Bi B Br 5-chloro-4-(1-chloroethyl)-3-bromoheptane 6-ethyl-3-iodo-5-isopropyl-4-propylnonane 2-bromo-4-(1-bromoethyl)-1-chlorocyclopentane
More Examples 6 F Br Cl Cl Br Cl Cl Br Br Cl Br I 3-fluoro-2,4-dimethylpentane 1-bromo-2-chloro-4-ethylcyclopentane 3-bromo-5-chloro-4-ethylheptane 5-chloro-4-(1-chloroethyl)-3-bromoheptane 2-bromo-4-(1-bromoethyl)-1-chlorocyclopentane 6-ethyl-3-iodo-5-isopropyl-4-propylnonane

Systematic Common Names You may encounter common names where the alkyl groups is a substituent on halide. Useful only for small alkyl groups. CH3 CH3 CH3 CH3CHCH2-Br CH3CH2CH-Br CH3C-Br CH3 isobutyl bromide sec-butyl bromide tert-butyl bromide 2013 Pearson Education Inc
Systematic Common Names • You may encounter common names where the alkyl groups is a substituent on halide. • Useful only for small alkyl groups. CH3CHCH2 CH3 Br CH3CH2CH CH3 Br CH3C CH3 Br CH3 isobutyl bromide sec-butyl bromide tert-butyl bromide 7 © 2013 Pearson Education Inc

Common Names of Halides CH2X2 is called methylene halide. 。CHX3 is a haloform. CX is carbon tetrahalide. Common halogenated solvents: CH,Cl,is methylene chloride. CHCl2 is chloroform. CCla is carbon tetrachloride. 8 2013 Pearson Education Inc
Common Names of Halides • CH2X2 is called methylene halide. • CHX3 is a haloform. • CX4 is carbon tetrahalide. • Common halogenated solvents: CH2Cl2 is methylene chloride. CHCl3 is chloroform. CCl4 is carbon tetrachloride. 8 © 2013 Pearson Education Inc

Alkyl Halides Classification Methyl halides:Halide is attached to a methyl group. Primary alkyl halide:Carbon to which halogen is bonded is attached to only one other carbon. Secondary alkyl halide:Carbon to which halogen is bonded is attached to two other carbons. Tertiary alkyl halide:Carbon to which halogen is bonded is attached to three other carbons. 2013 Pearson Education Inc. 9
Alkyl Halides Classification • Methyl halides: Halide is attached to a methyl group. • Primary alkyl halide: Carbon to which halogen is bonded is attached to only one other carbon. • Secondary alkyl halide: Carbon to which halogen is bonded is attached to two other carbons. • Tertiary alkyl halide: Carbon to which halogen is bonded is attached to three other carbons. 9 © 2013 Pearson Education Inc
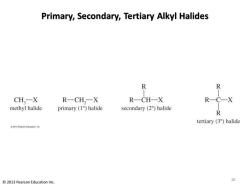
Primary,Secondary,Tertiary Alkyl Halides R CH,-X R一CH,一X R一CH-X methyl halide primary (1)halide secondary (2)halide R tertiary (3)halide 2013 Peurson Educaten.ine 2013 Pearson Education Inc. 10
Primary, Secondary, Tertiary Alkyl Halides 10 © 2013 Pearson Education Inc
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 22 Condensations and Alpha Substitutions of Carbonyl Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 21 Part 1 - Structure and Properties of Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 11 Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Reaction Mechanism and Synthesis Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 01 Structure and Bonding.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 02 Polar Covalent Bonds; Acids and Bases.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 03 Organic Compounds - Alkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 04 Organic Compounds - Cycloalkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 05 Stereochemistry at Tetrahedral Centers.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 06 An Overview of Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 07 Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 08 Reactions of Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 09 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 11 Structure Determination - Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 10 Structure Determination - Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 12 Organohalides - Nucleophilic Substitutions and Eliminations.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 13 Alcohols, Phenols, and Thiols; Ethers and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 14 Aldehydes and Ketones - Nucleophilic Additions Reactions.ppt
