《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 02 Polar Covalent Bonds; Acids and Bases

CNGNGE JOHN MCMURRY CHAPTER 2 Polar Covalent Bonds; Acids and Bases Organic Chemistry with Biological Applications
CHAPTER 2 Polar Covalent Bonds; Acids and Bases

2-1 Polar Covalent Bonds: Electronegativity Chemical bonds lonic bonds Bond in sodium chloride is ionic -Sodium transfers an electron to chlorine to give Na+and Cl- lons held together by electrostatic attractions between unlike charges Nonpolar Covalent bonds Two electrons are shared equally by the two bonding atoms Carbon-carbon bond in ethane Symmetrical electron distribution in the bond Most bonds are neither fully ionic or covalent
Chemical bonds ▪ Ionic bonds ▪ Bond in sodium chloride is ionic ▪ Sodium transfers an electron to chlorine to give Na+ and Cl- ▪ Ions held together by electrostatic attractions between unlike charges ▪ Nonpolar Covalent bonds ▪ Two electrons are shared equally by the two bonding atoms ▪ Carbon-carbon bond in ethane ▪ Symmetrical electron distribution in the bond Most bonds are neither fully ionic or covalent 2-1 Polar Covalent Bonds: Electronegativity
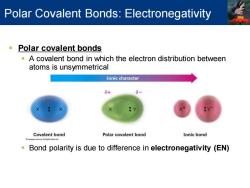
Polar Covalent Bonds:Electronegativity Polar covalent bonds A covalent bond in which the electron distribution between atoms is unsymmetrical Ionic character 8+ Covalent bond Polar covalent bond Ionic bond Bond polarity is due to difference in electronegativity (EN)
▪ Polar covalent bonds ▪ A covalent bond in which the electron distribution between atoms is unsymmetrical ▪ Bond polarity is due to difference in electronegativity (EN) Polar Covalent Bonds: Electronegativity
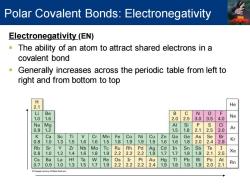
Polar Covalent Bonds:Electronegativity Electronegativity(EN) The ability of an atom to attract shared electrons in a covalent bond Generally increases across the periodic table from left to right and from bottom to top 2.1 He i Be B C N 1. 6 2.0 2.5 3.0 3.5 4.0 Ne N Mg Al i P CI 0. 2 1.5 1. 2.5 Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br . 0 1.3 1.5 1.6 1.6 5 1.8 9 1.9 9 6 1.6 1.8 2.0 2.4 2.8 Kr Rb Sr Zr Nb Mo Ru 22 22 铝 Cd In Sn Sb Te 8 1 1.2 4 6 8 1.9 2 7 7 8 9 2.1 2.5 Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At 0.7 .9 1.0 1.3 1.5 1.7 1.9 2.2 2.2 2.22.4 1.9 1.81.9 1.9 2.0 2.1 Rn
Electronegativity (EN) ▪ The ability of an atom to attract shared electrons in a covalent bond ▪ Generally increases across the periodic table from left to right and from bottom to top Polar Covalent Bonds: Electronegativity

Electronegativity and Bond Types Bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent Bonds between atoms whose electronegativities differ by 0.5 to 2.0 are polar covalent Bonds between atoms whose electronegativities differ by more than 2.0 are largely ionic Carbon hydrogen bonds are nonpolar.Bonds between carbon (EN 2.5)and more electronegative elements,such as oxygen (EN 3.5)and nitrogen (EN 3.0)are polar covalent bonds with the bonding electrons drawn towards the more electronegative atoms
▪ Bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent ▪ Bonds between atoms whose electronegativities differ by 0.5 to 2.0 are polar covalent ▪ Bonds between atoms whose electronegativities differ by more than 2.0 are largely ionic Carbon hydrogen bonds are nonpolar. Bonds between carbon (EN = 2.5) and more electronegative elements, such as oxygen (EN = 3.5) and nitrogen (EN = 3.0) are polar covalent bonds with the bonding electrons drawn towards the more electronegative atoms Electronegativity and Bond Types
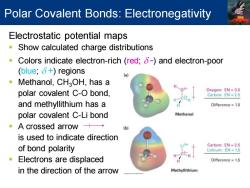
Polar Covalent Bonds:Electronegativity Electrostatic potential maps Show calculated charge distributions Colors indicate electron-rich (red;8-)and electron-poor (blue;δ+)regions (a Methanol,CHaOH,has a polar covalent C-O bond, Oxygen:EN=3.5 Carbon:EN=2.5 H and methyllithium has a Difference=1.0 polar covalent C-Li bond Methanol A crossed arrow+→ is used to indicate direction Carbon:EN 2.5 of bond polarity Lithium:EN 1.0 Electrons are displaced Difference=1.5 in the direction of the arrow Methyllithium
Electrostatic potential maps ▪ Show calculated charge distributions ▪ Colors indicate electron-rich (red; d -) and electron-poor (blue; d +) regions ▪ Methanol, CH3OH, has a polar covalent C-O bond, and methyllithium has a polar covalent C-Li bond ▪ A crossed arrow is used to indicate direction of bond polarity ▪ Electrons are displaced in the direction of the arrow Polar Covalent Bonds: Electronegativity

Polar Covalent Bonds:Electronegativity An atom's ability to polarize a bond is known as the inductive effect Inductive effect The electron-attracting or electron-withdrawing effect transmitted through o bonds.Electronegative elements have an electron-withdrawing inductive effect Metals inductively donate electrons Reactive nonmetals inductively withdraw electrons Inductive effects play a major role in understanding chemical reactivity
An atom’s ability to polarize a bond is known as the inductive effect Inductive effect ▪ The electron-attracting or electron-withdrawing effect transmitted through s bonds. Electronegative elements have an electron-withdrawing inductive effect ▪ Metals inductively donate electrons ▪ Reactive nonmetals inductively withdraw electrons ▪ Inductive effects play a major role in understanding chemical reactivity Polar Covalent Bonds: Electronegativity

2-2 Polar Covalent Bonds:Dipole Moments Molecules as a whole are often polar 日 Molecular polarity results from the vector summation of all individual bond polarities and lone-pair contributions in the molecule Strongly polar substances are soluble in polar solvents like water Dipole moment ( Magnitude of charge Q at either end of molecular dipole times distance r between charges u=oxr,in debyes (D) 1D=3.336×10-30 coulomb meter(C·m) A measure of the net polarity of a molecule Arises when the centers of mass of positive and negative charges within a molecule do not coincide
Molecules as a whole are often polar ▪ Molecular polarity results from the vector summation of all individual bond polarities and lone-pair contributions in the molecule ▪ Strongly polar substances are soluble in polar solvents like water Dipole moment () ▪ Magnitude of charge Q at either end of molecular dipole times distance r between charges ▪ = Q r, in debyes (D) 1 D = 3.336 10−30 coulomb meter (C • m) ▪ A measure of the net polarity of a molecule ▪ Arises when the centers of mass of positive and negative charges within a molecule do not coincide 2-2 Polar Covalent Bonds: Dipole Moments
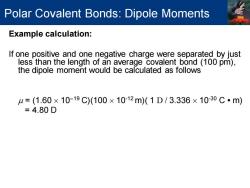
Polar Covalent Bonds:Dipole Moments Example calculation: If one positive and one negative charge were separated by just less than the length of an average covalent bond (100 pm), the dipole moment would be calculated as follows =(1.60×10-19C)(100×1012m)(1D/3.336×10-30C·m) =4.80D
Example calculation: If one positive and one negative charge were separated by just less than the length of an average covalent bond (100 pm), the dipole moment would be calculated as follows = (1.60 10−19 C)(100 10-12 m)( 1 D / 3.336 10-30 C • m) = 4.80 D Polar Covalent Bonds: Dipole Moments
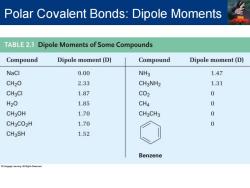
Polar Covalent Bonds:Dipole Moments TABLE2.1 Dipole Moments of Some Compounds Compound Dipole moment(D) Compound Dipole moment(D) NaCl 9.00 NH3 1.47 CH20 2.33 CH3NH2 1.31 CH3Cl 1.87 C02 0 H20 1.85 CH4 0 CH3OH 1.70 CH3CH3 0 CH3CO2H 1.70 0 CH3SH 1.52 Benzene
Polar Covalent Bonds: Dipole Moments
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 01 Structure and Bonding.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Reaction Mechanism and Synthesis Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 22 Condensations and Alpha Substitutions of Carbonyl Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 21 Part 1 - Structure and Properties of Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 03 Organic Compounds - Alkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 04 Organic Compounds - Cycloalkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 05 Stereochemistry at Tetrahedral Centers.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 06 An Overview of Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 07 Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 08 Reactions of Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 09 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 11 Structure Determination - Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 10 Structure Determination - Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 12 Organohalides - Nucleophilic Substitutions and Eliminations.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 13 Alcohols, Phenols, and Thiols; Ethers and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 14 Aldehydes and Ketones - Nucleophilic Additions Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 15 Carboxylic Acids and Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 16 Carboxylic Acid Derivatives - Nucleophilic Acyl Substitution Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 17 Carbonyl Alpha-Substitution and Condensation Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 18 Amines and Heterocycles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 19 Biomolecules - Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 20 Amino Acid Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 21 Biomolecules - Carbohydrates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 22 Carbohydrate Metabolism.ppt
