《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds

Organic Chemistry, ORGANIC 8th Edition CHEMISTRY L.G.Wade,Jr. Chapter 16 Lecture Aromatic Compounds 2013 Pearson Education,Inc
© 2013 Pearson Education, Inc. Chapter 16 1 Chapter 16 Lecture Organic Chemistry, 8 th Edition L. G. Wade, Jr. Aromatic Compounds © 2013 Pearson Education, Inc
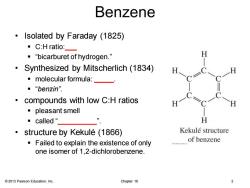
Benzene Isolated by Faraday (1825) ·C:H ratio:- ·“bicarburet of hydrogen.” Synthesized by Mitscherlich(1834) 。molecular formula:_— ■“benzin” compounds with low C:H ratios ·pleasant smell ·called“_ H structure by Kekule (1866) Kekule structure Failed to explain the existence of only of benzene one isomer of 1,2-dichlorobenzene. 2013 Pearson Education,Inc. Chapter 16 2
© 2013 Pearson Education, Inc. Chapter 16 2 Benzene • Isolated by Faraday (1825) ▪ C:H ratio:___ ▪ “bicarburet of hydrogen.” • Synthesized by Mitscherlich (1834) ▪ molecular formula: _____. ▪ “benzin”. • compounds with low C:H ratios ▪ pleasant smell ▪ called “__________”. • structure by Kekulé (1866) ▪ Failed to explain the existence of only one isomer of 1,2-dichlorobenzene
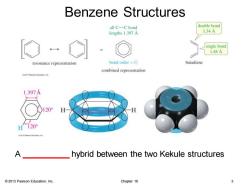
Benzene Structures all C-C bond double bond lengths 1.397A 134A single bond 1.48A resonance representation bond order =1 butadiene combined representation o20特earsonEdo 1.397A H120° A hybrid between the two Kekule structures 2013 Pearson Education,Inc. Chapter 16 3
© 2013 Pearson Education, Inc. Chapter 16 3 Benzene Structures A __________ hybrid between the two Kekule structures
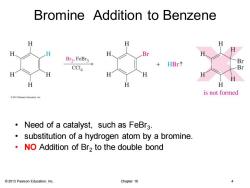
Bromine Addition to Benzene H H H H Br Br2,FeBr3 Br HBr个 CCI4 Br H H H H H H H is not formed 2013 Pearson Education.Inc. Need of a catalyst,such as FeBr3. substitution of a hydrogen atom by a bromine. NO Addition of Br2 to the double bond 2013 Pearson Education,Inc. Chapter 16 4
© 2013 Pearson Education, Inc. Chapter 16 4 Bromine Addition to Benzene • Need of a catalyst, such as FeBr3 . • substitution of a hydrogen atom by a bromine. • NO Addition of Br2 to the double bond
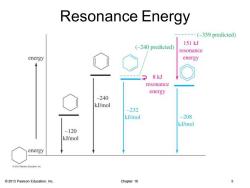
Resonance Energy ---(-359 predicted) 151kJ (-240 predicted) resonance energy energy 8kJ resonance energy -240 kJ/mol -232 kJ/mol -208 kJ/mol -120 kJ/mol energy 2013 Pearson Education,Inc. Chapter 16 5
© 2013 Pearson Education, Inc. Chapter 16 5 Resonance Energy
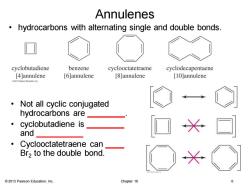
Annulenes hydrocarbons with alternating single and double bonds cyclobutadiene benzene cyclooctatetraene cyclodecapentaene [4]annulene [6]annulene [8]annulene [10]annulene 013 Pearson Education.Ine. Not all cyclic conjugated hydrocarbons are ● cyclobutadiene is and ● Cyclooctatetraene can Br2 to the double bond. 2013 Pearson Education,Inc. Chapter 16
© 2013 Pearson Education, Inc. Chapter 16 6 Annulenes • hydrocarbons with alternating single and double bonds. • Not all cyclic conjugated hydrocarbons are ________. • cyclobutadiene is ________ and __________ • Cyclooctatetraene can ____ Br2 to the double bond
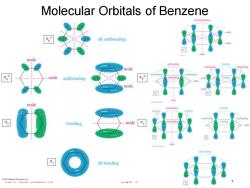
Molecular Orbitals of Benzene all antiboeding all antibonding node node node antibonding node node bonding node all bonding
© 2013 Pearson Education, Inc. Chapter 16 7 Molecular Orbitals of Benzene
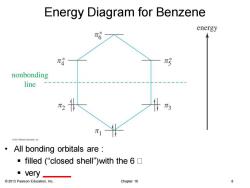
Energy Diagram for Benzene energy π哈 π4 nonbonding line π1 02013代arson Educaton nc All bonding orbitals are ·filled(“closed shell"with the6☐ very 2013 Pearson Education,Inc. Chapter 16 8
© 2013 Pearson Education, Inc. Chapter 16 8 Energy Diagram for Benzene • All bonding orbitals are : ▪ filled (“closed shell”)with the 6 ▪ very ______
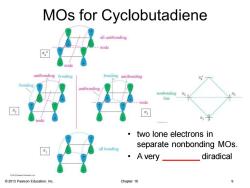
MOs for Cyclobutadiene all antibonding node node antibonding bonding bonding antibonding antibonding nonbonding line node ·two lone electrons in all bonding separate nonbonding MOs. ·Avey_ diradical e2接Prinon Eacaon Inc 2013 Pearson Education,Inc. Chapter 16 9
© 2013 Pearson Education, Inc. Chapter 16 9 MOs for Cyclobutadiene • two lone electrons in separate nonbonding MOs. • A very ________ diradical
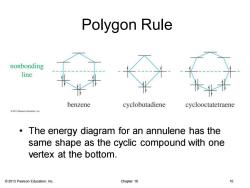
Polygon Rule nonbonding line benzene cyclobutadiene cyclooctatetraene 2013 Pearson Educaton.inc The energy diagram for an annulene has the same shape as the cyclic compound with one vertex at the bottom. 2013 Pearson Education,Inc. Chapter 16 0
© 2013 Pearson Education, Inc. Chapter 16 10 Polygon Rule • The energy diagram for an annulene has the same shape as the cyclic compound with one vertex at the bottom
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 22 Condensations and Alpha Substitutions of Carbonyl Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 21 Part 1 - Structure and Properties of Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Reaction Mechanism and Synthesis Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 01 Structure and Bonding.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 02 Polar Covalent Bonds; Acids and Bases.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 03 Organic Compounds - Alkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 04 Organic Compounds - Cycloalkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 05 Stereochemistry at Tetrahedral Centers.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 06 An Overview of Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 07 Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 08 Reactions of Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 09 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 11 Structure Determination - Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 10 Structure Determination - Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 12 Organohalides - Nucleophilic Substitutions and Eliminations.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 13 Alcohols, Phenols, and Thiols; Ethers and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 14 Aldehydes and Ketones - Nucleophilic Additions Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 15 Carboxylic Acids and Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 16 Carboxylic Acid Derivatives - Nucleophilic Acyl Substitution Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 17 Carbonyl Alpha-Substitution and Condensation Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 18 Amines and Heterocycles.ppt
