《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 03 Organic Compounds - Alkanes and Their Stereochemistry

CNGNGE JOHN MCMURRY CHAPTER 3 Organic Compounds: Alkanes and Their Stereochemistry EDITION Organic Chemistry with Biological Applications
CHAPTER 3 Organic Compounds: Alkanes and Their Stereochemistry

3-1 Functional Groups Functional group A group of atoms within a molecule that has a characteristic chemical behavior These structural features allow compounds to be classified into families
Functional group ▪ A group of atoms within a molecule that has a characteristic chemical behavior ▪ These structural features allow compounds to be classified into families 3-1 Functional Groups
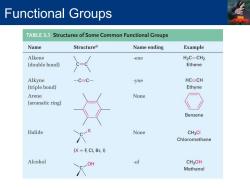
Functional Groups TABLE3.1 Structures of Some Common Functional Groups Name Structurea Name ending Example Alkene -ene H2C-CH2 (double bond) Ethene Alkyne C=C- -yne HC=CH (triple bond) Ethyne Arene None (aromatic ring) Benzene Halide None CH3CI Chloromethane (X F CI,Br,I) Alcohol OH -ol CH3OH Methanol
Functional Groups
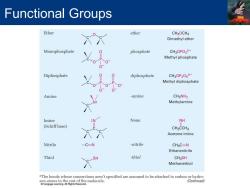
Functional Groups Ether ether CH30CH3 Dimethyl ether Monophosphate phosphate CH3OPO32- Methyl phosphate Diphosphate diphosphate CH3OP2063- Methyl diphosphate Amine -amine CH3NH2 Methylamine Imine None NH (Schiff base) CH3CCH3 Acetone imine Nitrile -C=N -nitrile CH3C=N Ethanenitrile Thiol SH thiol CH3SH Methanethiol "The bonds whose connections aren't specified are assumed to be attached to carbon or hydro- molecule. (Continued)
Functional Groups
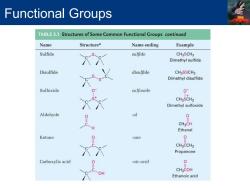
Functional Groups TABLE3.1 Structures of Some Common Functional Groups continued Name Structurea Name ending Example Sulfide sulfide CH3SCH3 Dimethyl sulfide Disulfide disulfide CHaSSCHa Dimethyl disulfide Sulfoxide sulfoxide 0 CH3SCH3 Dimethyl sulfoxide Aldehyde -al CH3CH Ethanal Ketone -one 0 CH3CCH3 Propanone Carboxylic acid -oic acid 0 CH3COH OH Ethanoic acid
Functional Groups
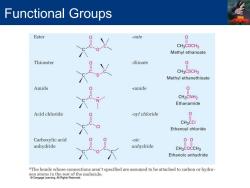
Functional Groups Ester -oate 0 CH3COCH3 Methyl ethanoate Thioester -thioate 0 CH3CSCH3 Methyl ethanethioate Amide -amide CH3CNH2 Ethanamide Acid chloride oyl chloride 0 CH3CCI Ethanoyl chloride Carboxylic acid -oic 00 anhydride anhydride CH3COCCH3 Ethanoic anhydride "The bonds whose connections aren't specified are assumed to be attached to carbon or hydro- molecule
Functional Groups
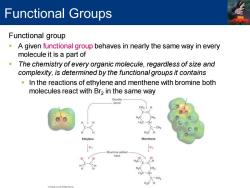
Functional Groups Functional group A given functional group behaves in nearly the same way in every molecule it is a part of The chemistry of every organic molecule,regardless of size and complexity,is determined by the functional groups it contains In the reactions of ethylene and menthene with bromine both molecules react with Br2 in the same way Double bond CH H2C H2C-CH C-CH3 Ethylene Menthene Bromine added here CH2 H2C-CH C-CH3
Functional group ▪ A given functional group behaves in nearly the same way in every molecule it is a part of ▪ The chemistry of every organic molecule, regardless of size and complexity, is determined by the functional groups it contains ▪ In the reactions of ethylene and menthene with bromine both molecules react with Br2 in the same way Functional Groups
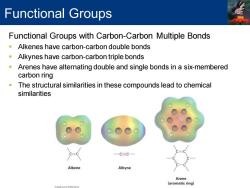
Functional Groups Functional Groups with Carbon-Carbon Multiple Bonds Alkenes have carbon-carbon double bonds -Alkynes have carbon-carbon triple bonds Arenes have alternating double and single bonds in a six-membered carbon ring The structural similarities in these compounds lead to chemical similarities 一C=C一 Alkene Alkyne Arene (aromatic ring)
Functional Groups with Carbon-Carbon Multiple Bonds ▪ Alkenes have carbon-carbon double bonds ▪ Alkynes have carbon-carbon triple bonds ▪ Arenes have alternating double and single bonds in a six-membered carbon ring ▪ The structural similarities in these compounds lead to chemical similarities Functional Groups

Functional Groups Functional Groups with Carbon Singly Bonded to an Electronegative Atom Example functional groups include alkyl halides (haloalkanes),alcohols, ethers,alkyl phosphates, amines,thiols,sulfides and disulfides C-O 。 Bonds are polar Alcohol Ether Phosphat Carbon bears a partial positive charge(+) Electronegative atom bears a partial negative charge() Amine Thiol Sulfide Disulfide
Functional Groups with Carbon Singly Bonded to an Electronegative Atom Functional Groups Example functional groups include alkyl halides (haloalkanes), alcohols, ethers, alkyl phosphates, amines, thiols, sulfides and disulfides • Bonds are polar • Carbon bears a partial positive charge (d+) • Electronegative atom bears a partial negative charge (d-)

Functional Groups Functional Groups with a Carbon-Oxygen Double Bond (Carbonyl Groups) Carbonyl group ·C=O -Carbonyl carbon bears a partial positive charge() Carbonyl oxygen bears a partial negative charge() Acetone-a typical carbonyl compound
▪ Carbonyl group ▪ C=O ▪ Carbonyl carbon bears a partial positive charge (d+) ▪ Carbonyl oxygen bears a partial negative charge (d-) Functional Groups with a Carbon-Oxygen Double Bond (Carbonyl Groups) Functional Groups
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 02 Polar Covalent Bonds; Acids and Bases.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 01 Structure and Bonding.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Reaction Mechanism and Synthesis Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 22 Condensations and Alpha Substitutions of Carbonyl Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 21 Part 1 - Structure and Properties of Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 04 Organic Compounds - Cycloalkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 05 Stereochemistry at Tetrahedral Centers.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 06 An Overview of Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 07 Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 08 Reactions of Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 09 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 11 Structure Determination - Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 10 Structure Determination - Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 12 Organohalides - Nucleophilic Substitutions and Eliminations.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 13 Alcohols, Phenols, and Thiols; Ethers and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 14 Aldehydes and Ketones - Nucleophilic Additions Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 15 Carboxylic Acids and Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 16 Carboxylic Acid Derivatives - Nucleophilic Acyl Substitution Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 17 Carbonyl Alpha-Substitution and Condensation Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 18 Amines and Heterocycles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 19 Biomolecules - Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 20 Amino Acid Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 21 Biomolecules - Carbohydrates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 22 Carbohydrate Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 23 Biomolecules - Lipids and Their Metabolism.ppt
