《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 19 Biomolecules - Amino Acids, Peptides, and Proteins

CNGNGE JOHN MCMURRY CHAPTER 19 Biomolecules: Amino Acids,Peptides, and Proteins T H I R D E DI TION Organic Chemistry with Biological Applications
CHAPTER 19 Biomolecules: Amino Acids, Peptides, and Proteins
Amino Acids,Peptides,and Proteins Proteins Occur in every living organism Are of many different types Have many different biological functions Keratin of skin and fingernails -Fibroin of silk and spider webs Estimated 50,000 to 70,000 enzymes that catalyze the biological functions of the human body Made up of many amino acids linked together
Proteins ▪ Occur in every living organism ▪ Are of many different types ▪ Have many different biological functions ▪ Keratin of skin and fingernails ▪ Fibroin of silk and spider webs ▪ Estimated 50,000 to 70,000 enzymes that catalyze the biological functions of the human body ▪ Made up of many amino acids linked together Amino Acids, Peptides, and Proteins
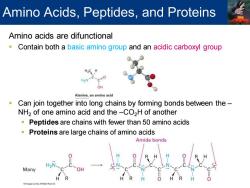
Amino Acids,Peptides,and Proteins Amino acids are difunctional Contain both a basic amino group and an acidic carboxyl group H2N c=0 OH Alanine,an amino acid Can join together into long chains by forming bonds between the- NH2 of one amino acid and the-CO2H of another Peptides are chains with fewer than 50 amino acids Proteins are large chains of amino acids Amide bonds Many
Amino acids are difunctional ▪ Contain both a basic amino group and an acidic carboxyl group ▪ Can join together into long chains by forming bonds between the – NH2 of one amino acid and the –CO2H of another ▪ Peptides are chains with fewer than 50 amino acids ▪ Proteins are large chains of amino acids Amino Acids, Peptides, and Proteins
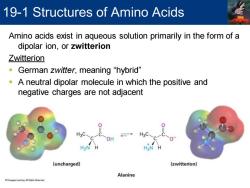
19-1 Structures of Amino Acids Amino acids exist in aqueous solution primarily in the form of a dipolar ion,or zwitterion Zwitterion German zwitter,,meaning“hybrid” A neutral dipolar molecule in which the positive and negative charges are not adjacent H3C. → H3C OH H2>N H H3N (uncharged) (zwitterion) Alanine
Amino acids exist in aqueous solution primarily in the form of a dipolar ion, or zwitterion Zwitterion ▪ German zwitter, meaning “hybrid” ▪ A neutral dipolar molecule in which the positive and negative charges are not adjacent 19-1 Structures of Amino Acids
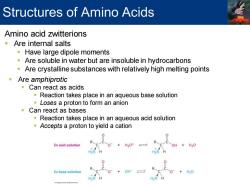
Structures of Amino Acids Amino acid zwitterions Are internal salts Have large dipole moments Are soluble in water but are insoluble in hydrocarbons Are crystalline substances with relatively high melting points Are amphiprotic Can react as acids Reaction takes place in an aqueous base solution Loses a proton to form an anion Can react as bases Reaction takes place in an aqueous acid solution Accepts a proton to yield a cation 0 In acid solution +H20 In base solution H20
Amino acid zwitterions ▪ Are internal salts ▪ Have large dipole moments ▪ Are soluble in water but are insoluble in hydrocarbons ▪ Are crystalline substances with relatively high melting points ▪ Are amphiprotic ▪ Can react as acids ▪ Reaction takes place in an aqueous base solution ▪ Loses a proton to form an anion ▪ Can react as bases ▪ Reaction takes place in an aqueous acid solution ▪ Accepts a proton to yield a cation Structures of Amino Acids
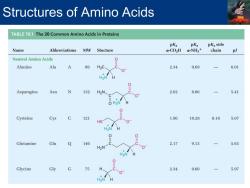
Structures of Amino Acids TABLE19.1 The 20 Common Amino Acids in Proteins pKa pKa pKa side Name Abbreviations MW Stucture a-CO2H a-NH3+ chain pl Neutral Amino Acids 0 Alanine Ala A 89 H3C、 2.34 9.69 6.01 0 H3N H O Asparagine Asn N 132 H2N 2.02 8.80 5.41 O H3N 0 Cysteine Cys C 121 1.96 10.28 8.18 5.07 HS 0 Glutamine Gln Q 146 2.17 9.13 5.65 H2N Glycine Gly G 75 2.34 9.60 5.97 0
Structures of Amino Acids
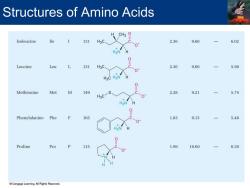
Structures of Amino Acids Isoleucine 131 2.36 9.60 6.02 0 0 Leucine Leu 131 HgC 2.36 9.60 一 5.98 Methionine Met M 149 2.28 9.21 一 5.74 H3C Phenylalanine Phe 165 1.83 9.13 一 5.48 Proline Pro 115 1.99 10.60 6.30 Cengage Learning.All Rights Reserved
Structures of Amino Acids
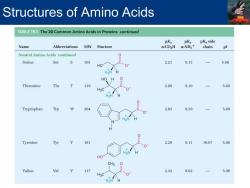
Structures of Amino Acids TABLE 19.1 The 20 Common Amino Acids in Proteins continued pKa pKa pKa side Name Abbreviations MW Stucture a-CO2H a-NH3+ chain Neutral Amino Acids continued Serine Ser 105 2.21 9.15 5.68 HO H HO H Threonine Thr 119 2.09 9.10 5.60 H3C H3 Tryptophan Trp 2 204 2.83 9.39 5.89 Tyrosine Tyr 181 2.20 9.11 10.07 5.66 HO CH3 Valine Val 117 2.32 9.62 5.96 H3C 0
Structures of Amino Acids
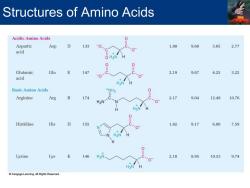
Structures of Amino Acids Acidic Amino Acids Aspartic Asp 0 133 0 1.88 9.60 3.65 2.77 acid O H3N Glutamic Glu E 147 2.19 9.67 4.25 3.22 acid H3N Basic Amino Acids +NH2 Arginine Arg R 174 2.17 9.04 12.48 10.76 H>N Histidine His H 155 1.82 9.17 6.00 7.59 H3 Lysine Lys 146 H3 2.18 8.95 10.53 9.74 Cengage Learning.All Rights Reserved
Structures of Amino Acids
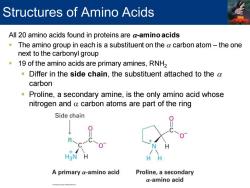
Structures of Amino Acids All 20 amino acids found in proteins are a-amino acids The amino group in each is a substituent on the a carbon atom-the one next to the carbonyl group 19 of the amino acids are primary amines,RNH2 Differ in the side chain,the substituent attached to the a carbon Proline,a secondary amine,is the only amino acid whose nitrogen and a carbon atoms are part of the ring Side chain H3N H HH A primary a-amino acid Proline,a secondary a-amino acid
All 20 amino acids found in proteins are a-amino acids ▪ The amino group in each is a substituent on the a carbon atom – the one next to the carbonyl group ▪ 19 of the amino acids are primary amines, RNH2 ▪ Differ in the side chain, the substituent attached to the a carbon ▪ Proline, a secondary amine, is the only amino acid whose nitrogen and a carbon atoms are part of the ring Structures of Amino Acids
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 18 Amines and Heterocycles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 17 Carbonyl Alpha-Substitution and Condensation Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 16 Carboxylic Acid Derivatives - Nucleophilic Acyl Substitution Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 15 Carboxylic Acids and Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 14 Aldehydes and Ketones - Nucleophilic Additions Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 13 Alcohols, Phenols, and Thiols; Ethers and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 12 Organohalides - Nucleophilic Substitutions and Eliminations.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 10 Structure Determination - Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 11 Structure Determination - Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 09 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 08 Reactions of Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 07 Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 06 An Overview of Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 05 Stereochemistry at Tetrahedral Centers.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 04 Organic Compounds - Cycloalkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 03 Organic Compounds - Alkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 02 Polar Covalent Bonds; Acids and Bases.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 01 Structure and Bonding.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Reaction Mechanism and Synthesis Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 20 Amino Acid Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 21 Biomolecules - Carbohydrates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 22 Carbohydrate Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 23 Biomolecules - Lipids and Their Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 24 Biomolecules - Nucleic Acids and Their Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 25 Secondary Metabolites - An Introduction to Natural Products Chemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 26 Orbitals and Organic Chemistry - Pericyclic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 27 Synthetic Polymers.ppt
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 01 Introduction.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 02 Structure and Properties.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 03 Alkanes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 04 Rates & Kinetics.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 05 Stereochemistry.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 06 Alkyl Halides.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 07 Structure and Synthesis of Alkenes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 08 Reactions of Alkenes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 09 Alkynes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 10 Synthesis and Structure of Alcohols.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 11 Reactions of Alcohols.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.pdf
