《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 09 Alkynes

Alkynes Alkynes or acetylenes are compounds that contain a carbon-carbon triple bond. E.g. H-C=C-H CHgCH2-C=C-H HgC-C=C-CH3 acetylene The triple bond results in a molecular formula of C H2n2 Ethane CH 0 elements of unsaturation Ethene CH 1 element of unsaturation Ethyne C2H2 2 elements of unsaturation The triple bond contributes two elements of unsaturation. Nomenclature of Alkynes IUPAC nomenclature is similar to that for alkenes,except the-ane ending is replaced with-yne. The chain is numbered from the end closest to the triple bond. CH, Br H-C=C-H CH,-C=C-H CH,-C=C-CH,CH,-CH-C=C-CH,-CH-CH, old IUPAC name: ethyne propyne 2-butyne 6-bromo-2-methyl-3-heptyne new IUPAC name: ethyne propyne but-2-yne 6-bromo-2-methylhept-3-yne Ch09 Alkynes (landscape) Page I
Ch09 Alkynes (landscape) Page 1 Alkynes Alkynes or acetylenes are compounds that contain a carbon–carbon triple bond. E.g. The triple bond results in a molecular formula of CnH2n-2 Ethane C2H6 0 elements of unsaturation Ethene C2H4 1 element of unsaturation Ethyne C2H2 2 elements of unsaturation The triple bond contributes two elements of unsaturation. Nomenclature of Alkynes IUPAC nomenclature is similar to that for alkenes, except the –ane ending is replaced with –yne. The chain is numbered from the end closest to the triple bond. H C C acetylene H CH3CH2 C C H H3C C C CH3

When additional functional groups are present,the suffixes are combined. H,C-C-C=C-CH, CH,一CH-C=C-H CH,=CH一CH2一C=CH CH, OH old IUPAC name: 2-methyl-I-penten-3-yne 3-butyn-2-ol 1-penten-4-yne new IUPAC name: 2-methylpent-1-en-3-yne but-3-yn-2-ol pent-1-en-4-yne 2013 Pearson Education,Inc Terminal and Internal Alkynes The position of the triple bond can alter the reactivity of the alkyne. Compounds with triple bonds at the end of a molecule are called terminal alkynes.(Terminal C-H groups are called acetylenic hydrogens). acetylenic hydrogen (no acetylenic hydrogen) HC=C一CH,CH CH3一C=C一CH3 but-1-yne,a terminal alkyne but-2-yne,an internal alkyne If the triple bond is flanked by alkyl groups on both sides it is an internal alkyne. Ch09 Alkynes (landscape) Page 2
Ch09 Alkynes (landscape) Page 2 When additional functional groups are present, the suffixes are combined. Terminal and Internal Alkynes The position of the triple bond can alter the reactivity of the alkyne. Compounds with triple bonds at the end of a molecule are called terminal alkynes. (Terminal C-H groups are called acetylenic hydrogens). If the triple bond is flanked by alkyl groups on both sides it is an internal alkyne
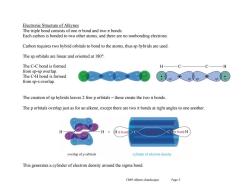
Electronic Structure of Alkynes The triple bond consists of one o bond and two nt bonds. Each carbon is bonded to two other atoms,and there are no nonbonding electrons Carbon requires two hybrid orbitals to bond to the atoms,thus sp hybrids are used. The sp orbitals are linear and oriented at 180 The C-C bond is formed H from sp-sp overlap. The C-H bond is formed from sp-s overlap. The creation of sp hybrids leaves 2 free p orbitals-these create the two nt bonds The p orbitals overlap just as for an alkene,except there are two nt bonds at right angles to one another. Hc bond o bond H overlap of p orbitals cylinder of eleetron density This generates a cylinder of electron density around the sigma bond. Ch09 Alkynes (landscape) Page 3
Ch09 Alkynes (landscape) Page 3 Electronic Structure of Alkynes The triple bond consists of one bond and two bonds. Each carbon is bonded to two other atoms, and there are no nonbonding electrons. Carbon requires two hybrid orbitals to bond to the atoms, thus sp hybrids are used. The sp orbitals are linear and oriented at 180°. The C-C bond is formed from sp-sp overlap. The C-H bond is formed from sp-s overlap. The creation of sp hybrids leaves 2 free p orbitals – these create the two bonds. The p orbitals overlap just as for an alkene, except there are two bonds at right angles to one another. This generates a cylinder of electron density around the sigma bond

The C-C bond length for ethyne is 1.20A which is shorter than ethane(1.54A)and ethene(1.33A) The C-H bond length in ethyne is 1.06A which is also shorter than in ethane (1.09A)or ethene(1.08A). This is because the C-H bond contains more s character(sp'->sp->sp)which gives stronger bonds. 1.54A 1.33A 1.20A H H H C≡C一H H H H 1.09 1.08A 1.06A ethane ethene ethyne Ch09 Alkynes (landscape) Page4
Ch09 Alkynes (landscape) Page 4 The C-C bond length for ethyne is 1.20Å which is shorter than ethane (1.54Å) and ethene (1.33Å). The C-H bond length in ethyne is 1.06Å which is also shorter than in ethane (1.09Å) or ethene (1.08Å). This is because the C-H bond contains more s character (sp3sp 2sp) which gives stronger bonds

Acidity of Alkynes Terminal alkynes are acidic,the end hydrogen can be removed as a proton by a strong base. E.g CH,CH2一C=C一H+Na+NH, CHCH,-C=C:-Na+NH but-1-yne,a terminal alkyne sodium amide sodium butynide C=C一H NaNH2 C=C:-Na+NH, cyclohexylacetylene sodium amide sodium cyclohexylacetylide CH,一C=C-CH NaNH2> no reaction (no acetylenic proton) but-2-yne,an internal alkyne Synthesis of Alkynes The carbanions produced are called acetylides,and are strong nucleophiles. R-C=C: S2, R-C=C-R'十X (R-X must be a primary alkyl halide) They can react in SN2 displacements of halides or tosylates from primary unhindered substrates to produce internal alkynes. Ch09 Alkynes(landscape) Page 5
Ch09 Alkynes (landscape) Page 5 Acidity of Alkynes Terminal alkynes are acidic, the end hydrogen can be removed as a proton by a strong base. E.g. Synthesis of Alkynes The carbanions produced are called acetylides, and are strong nucleophiles. They can react in SN2 displacements of halides or tosylates from primary unhindered substrates to produce internal alkynes
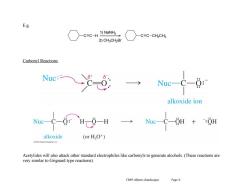
E.g. C≡C-H 1)NaNH2 C≡C-CH2CH 2)CH3CH2Br Carbonyl Reactions Nuc: Nuc- 0: alkoxide ion alkoxide (or HO+) Acetylides will also attack other standard electrophiles like carbonyls to generate alcohols.(These reactions are very similar to Grignard type reactions) Ch09 Alkynes (landscape) Page6
Ch09 Alkynes (landscape) Page 6 E.g. Carbonyl Reactions Acetylides will also attack other standard electrophiles like carbonyls to generate alcohols. (These reactions are very similar to Grignard type reactions). C C H 1) NaNH2 2) CH3CH2Br C C CH2CH3
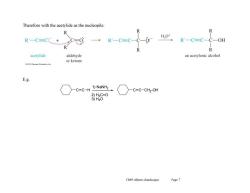
Therefore with the acetylide as the nucleopile: 个 HO* R'一C=C+ R'-C=C-C-0 R'一C=C-C一OH R acetylide aldehyde an acetylenic alcohol or ketone E.g. C=C-H 1)NaNH2 C=C-CH2-OH 2)H2C=O 3)H20 Ch09 Alkynes (landscape) Page 7
Ch09 Alkynes (landscape) Page 7 Therefore with the acetylide as the nucleopile: E.g. C C H 1) NaNH2 2) H2C=O 3) H2O C C CH2 -OH

Synthesis of Alkynes via Elimination In the same way that alkenes are produced by elimination of H-X,alkynes can be produced by elimination of 2 moles of H-X from a geminal or vicinal dihalide -HX R HKR三-R FAST H SLOW Strongly basic conditions(KOH or NaNH2,high temps)are required for this transformation,and often the molecule may not be stable to these conditions and may be destroyed or will rearrange. Ch09 Alkynes (landscape) Page 8
Ch09 Alkynes (landscape) Page 8 Synthesis of Alkynes via Elimination In the same way that alkenes are produced by elimination of H-X, alkynes can be produced by elimination of 2 moles of H-X from a geminal or vicinal dihalide. Strongly basic conditions (KOH or NaNH2, high temps) are required for this transformation, and often the molecule may not be stable to these conditions and may be destroyed or will rearrange
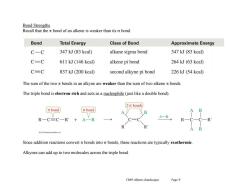
Bond Strengths Recall that the nt bond of an alkene is weaker than its o bond. Bond Total Energy Class of Bond Approximate Energy C-C 347 kJ(83 kcal) alkane sigma bond 347 kJ (83 kcal) C=C 611 kJ(146 kcal) alkene pi bond 264 kJ (63 kcal) CC 837 kJ(200 kcal) second alkyne pi bond 226 kJ(54 kcal) The sum of the two at bonds in an alkyne are weaker than the sum of two alkene nt bonds. The triple bond is electron rich and acts as a nucleophile (just like a double bond). 2 o bonds t bond [o bond A-P R一C=C-R'+A一B Since addition reactions convert bonds into o bonds,these reactions are typically exothermic. Alkynes can add up to two molecules across the triple bond. Ch09 Alkynes (landscape) Page 9
Ch09 Alkynes (landscape) Page 9 Bond Strengths Recall that the bond of an alkene is weaker than its bond. The sum of the two bonds in an alkyne are weaker than the sum of two alkene bonds. The triple bond is electron rich and acts as a nucleophile (just like a double bond). Since addition reactions convert bonds into bonds, these reactions are typically exothermic. Alkynes can add up to two molecules across the triple bond

Addition of Hydrogen Hydrogen in the presence of a catalyst will add twice to alkynes to generate alkanes. H H2,Pt H,Pt R-C=C一RM H H This reaction proceeds through a cis alkene intermediate,but cannot be stopped at this stage except with the use of a special catalyst. H-C=C-CH2CHs +2H2R,H-CH2-CH2- CH2CH3 but-1-yne butan (100% CH3-C=C-CH3 +2H2>CH3-CH2-CH2-CH3 but-2-yne butane (100%) The special catalyst is:Lindlar's catalyst This is a partially deactivated(poisoned)catalyst consisting of barium sulfate,palladium and quinoline(the poison). H2,Pd/BaSO4 R-C≡C-R' Quinoline,CH3OH R The hydrogens are delivered simultaneously to the same side of the alkyne,creating syn addition(cis alkenes). Ch09 Alkynes (landscape) Page 10
Ch09 Alkynes (landscape) Page 10 Addition of Hydrogen Hydrogen in the presence of a catalyst will add twice to alkynes to generate alkanes. This reaction proceeds through a cis alkene intermediate, but cannot be stopped at this stage except with the use of a special catalyst. The special catalyst is: Lindlar’s catalyst This is a partially deactivated (poisoned) catalyst consisting of barium sulfate, palladium and quinoline (the poison). The hydrogens are delivered simultaneously to the same side of the alkyne, creating syn addition (cis alkenes). R C C R' H R H R' H2 , Pd/BaSO4 Quinoline, CH3OH
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 08 Reactions of Alkenes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 07 Structure and Synthesis of Alkenes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 06 Alkyl Halides.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 05 Stereochemistry.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 04 Rates & Kinetics.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 03 Alkanes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 02 Structure and Properties.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 01 Introduction.pdf
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 27 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 26 Orbitals and Organic Chemistry - Pericyclic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 25 Secondary Metabolites - An Introduction to Natural Products Chemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 24 Biomolecules - Nucleic Acids and Their Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 23 Biomolecules - Lipids and Their Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 22 Carbohydrate Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 21 Biomolecules - Carbohydrates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 20 Amino Acid Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 19 Biomolecules - Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 18 Amines and Heterocycles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 17 Carbonyl Alpha-Substitution and Condensation Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 16 Carboxylic Acid Derivatives - Nucleophilic Acyl Substitution Reactions.ppt
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 10 Synthesis and Structure of Alcohols.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 11 Reactions of Alcohols.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 13 Nuclear Magnetic Resonance(NMR)Spectroscopy.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 14 Ethers and Epoxides.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 15 Conjugated Systems.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 16 Aromatic Compounds.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 17 Reactions of Aromatic Compounds.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 18 Ketones and Aldehydes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 19 Amines.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 20 Carboxylic Acids.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 21 Carboxylic acid Derivatives.pdf
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)CHM 201 Introduction and Review - Structure and Bonding.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Structure and Bonding of Organic Molecules.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alcohols-structure and synthesis 2.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Overview.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkyl Halides from Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkynes McMurry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Benzene and Aromaticity(2011).ppt
