《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 14 Ethers and Epoxides

Organic Chemistry II with Dr Roche Lecture Notes http://crab.rutgers.edu/~alroche Email alroche@crab.rutgers.edu Room Sci311 labs:Sci328/309/319) Phone 225-6166 Text (a)“Organic Chemistry'”Wade,4..8 Editions (b)Solution Manual,4....Eds (organic model kit) Do the problems in the book Learn as you go along Aim for understanding,not memorization Chl4 Ethers and Epoxides (landscape).docx Page 0
Ch14 Ethers and Epoxides (landscape).docx Page 0 Organic Chemistry II with Dr Roche Lecture Notes http://crab.rutgers.edu/~alroche Email alroche@crab.rutgers.edu Room Sci 311 (labs: Sci 328/309/319) Phone 225-6166 Text (a) “Organic Chemistry” Wade, 4….8th Editions (b) Solution Manual, 4…..8th Eds (organic model kit) Do the problems in the book Learn as you go along Aim for understanding, not memorization

Ethers and Epoxides Ethers are a class of compound of the general formula R-O-R'. R and R'can be alkyl or aryl Structure Ethers can be thought of as alkyl analogues of water HO-H RO-R Uses Since ethers are relatively unreactive and are strongly polar(due to the lone pairs on the oxygen),they are commonly used as solvents for organic reactions.(Diethyl ether and THF,the Grignard reaction). Ethers will often form complexes with molecules that have vacant orbitals,enabling'unstable'molecules to be used as reagents. E.g.Hydroboration uses BH:.THF ”g HHH Chl4 Ethers and Epoxides (landscape).docx Page I
Ch14 Ethers and Epoxides (landscape).docx Page 1 Ethers and Epoxides Ethers are a class of compound of the general formula R-O-R’. R and R’ can be alkyl or aryl. Structure Ethers can be thought of as alkyl analogues of water. Uses Since ethers are relatively unreactive and are strongly polar (due to the lone pairs on the oxygen), they are commonly used as solvents for organic reactions. (Diethyl ether and THF, the Grignard reaction). Ethers will often form complexes with molecules that have vacant orbitals, enabling ‘unstable’ molecules to be used as reagents. E.g. Hydroboration uses BH3.THF H O H R O R

Crown ethers are macrocyclic ethers,which help to solvate metal cations,and thus allow inorganic salts to dissolve in organic solvents. 12-crown-4 15-crown-5 18-crown-6 18-crown-6 EPM of 18-crown-6 solvates LI solvates Nat solvates K' with K+solvated 18-Crown-6 is the ideal size to incorporate a potassium ion,and allows organic solutions of ionic potassium salts to be prepared (purple benzene). Chl4 Ethers and Epoxides (landscape).docx Page2
Ch14 Ethers and Epoxides (landscape).docx Page 2 Crown ethers are macrocyclic ethers, which help to solvate metal cations, and thus allow inorganic salts to dissolve in organic solvents. 18-Crown-6 is the ideal size to incorporate a potassium ion, and allows organic solutions of ionic potassium salts to be prepared (purple benzene)
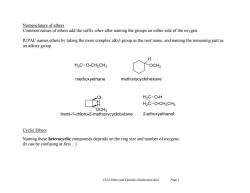
Nomenclature of ethers Common names of ethers add the suffix ether after naming the groups on either side of the oxygen. IUPAC names ethers by taking the more complex alkyl group as the root name,and naming the remaining part as an alkoxy group. H3C-O-CH2CH3 OCHa methoxyethane methoxycyclohexane H2C-O-H H2C-O-CH2CH3 OCH3 trans-1-chloro-2-methoxycyclobutane 2-ethoxyethanol Cyclic Ethers Naming these heterocyclic compounds depends on the ring size and number of oxygens (It can be confusing at first...) Chl4 Ethers and Epoxides (landscape).docx Page 3
Ch14 Ethers and Epoxides (landscape).docx Page 3 Nomenclature of ethers Common names of ethers add the suffix ether after naming the groups on either side of the oxygen. IUPAC names ethers by taking the more complex alkyl group as the root name, and naming the remaining part as an alkoxy group. Cyclic Ethers Naming these heterocyclic compounds depends on the ring size and number of oxygens. (It can be confusing at first…)

Epoxides These 3 membered rings are named using the term epoxy as a substituent H203H CH, CH-CH,一CH OCH, cis-2.3-epoxy-4-methoxyhexane (cis refers to the substituents,not the epoxide which must be cis/syn). Epoxides have considerable ring strain. Oxetanes These are four membered rings with one oxygen. CH3- 3 2 CH,CH CH H oxetane 2-ethyl-3,3-dimethyloxetane Oxetanes have ring strain,but not as much as epoxides. Chl4 Ethers and Epoxides (landscape).docx Page 4
Ch14 Ethers and Epoxides (landscape).docx Page 4 Epoxides These 3 membered rings are named using the term epoxy as a substituent. (cis refers to the substituents, not the epoxide which must be cis/syn). Epoxides have considerable ring strain. Oxetanes These are four membered rings with one oxygen. Oxetanes have ring strain, but not as much as epoxides
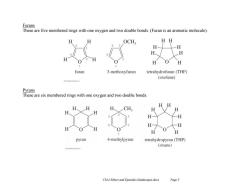
Furans These are five membered rings with one oxygen and two double bonds.(Furan is an aromatic molecule). H H OCH HH H H H furan 3-methoxyfuran tetrahydrofuran(THF) (oxolane) Pyrans These are six membered rings with one oxygen and two double bonds. HH H、H H、CH H H H H H- 夕 pyran 4-methylpyran tetrahydropyran(THP) (oxane) Chl4 Ethers and Epoxides (landscape).docx Page 5
Ch14 Ethers and Epoxides (landscape).docx Page 5 Furans These are five membered rings with one oxygen and two double bonds. (Furan is an aromatic molecule). Pyrans These are six membered rings with one oxygen and two double bonds
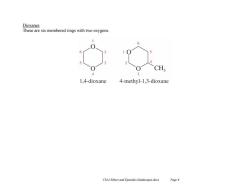
Dioxanes These are six membered rings with two oxygens CH 4 3 1,4-dioxane 4-methyl-1,3-dioxane Chl4 Ethers and Epoxides (landscape).docx Page 6
Ch14 Ethers and Epoxides (landscape).docx Page 6 Dioxanes These are six membered rings with two oxygens
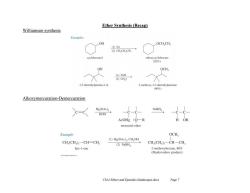
Ether Synthesis (Recap) Williamson synthesis Examples OH OCHCH, (1)Na ②CH,CH,0ms→ cyclohexanol (92% OH OCH, (1)NaH ②CH,I→ 3.3-dimethylpentan-2-ol 2-methoxy-3.3-dimethylpentane (90%) Alkoxymercuration-Demercuration C=C Hg(OAc) NaBH, ROH AcOHg :O一R H OR mercurial ether Example OCH, (1)Hg(OAc):CH,OH CH,(CH,一CH=CH, (2)NaBHa CH,(CH)-CH一CH hex-1-ene 2-methoxyhexane,80% (Markovnikov product) Chl4 Ethers and Epoxides (landscape).docx Page 7
Ch14 Ethers and Epoxides (landscape).docx Page 7 Ether Synthesis (Recap) Williamson synthesis Alkoxymercuration-Demercuration

Bimolecular Dehydration of Alcohols 2R-OH R-0-R H,O Examples H,S0140C 2 CH,OH CH,-O-CH3 +H,O methyl alcohol dimethyl ether (100%) H,S04,140C CH,CH,OH CHCH,-O-CH,CH H,O ethyl alcohol diethyl ether 88%) Chl4 Ethers and Epoxides (landscape).docx Page 8
Ch14 Ethers and Epoxides (landscape).docx Page 8 Bimolecular Dehydration of Alcohols

Reactions of Ethers Typically ethers are stable and chemically inert,although they can undergo two types of reaction(cleavage, oxidation). Cleavage Ethers are cleaved by H-Br and H-I,generating the corresponding alkyl halides. R-O-R'+excess H-X>R-X +R’X Ethers are stable to bases,but acidic conditions leads to the protonation of the ether oxygen,which then can undergo substitution reactions. -Br CH3CH2-Br CH2CH2-O-CH2CH3 +CH3CH2-OCH2CH3→ H3CH2-QH H-Br Br: H CHgCH2-Br CHaCH2O-H The alcohol produced reacts to generate a second molecule of alkyl halide. Chl4 Ethers and Epoxides (landscape).docx Page 9
Ch14 Ethers and Epoxides (landscape).docx Page 9 Reactions of Ethers Typically ethers are stable and chemically inert, although they can undergo two types of reaction (cleavage, oxidation). Cleavage Ethers are cleaved by H-Br and H-I, generating the corresponding alkyl halides. R-O-R’ + excess H-X R-X + R’-X Ethers are stable to bases, but acidic conditions leads to the protonation of the ether oxygen, which then can undergo substitution reactions. The alcohol produced reacts to generate a second molecule of alkyl halide
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 13 Nuclear Magnetic Resonance(NMR)Spectroscopy.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 11 Reactions of Alcohols.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 10 Synthesis and Structure of Alcohols.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 09 Alkynes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 08 Reactions of Alkenes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 07 Structure and Synthesis of Alkenes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 06 Alkyl Halides.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 05 Stereochemistry.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 04 Rates & Kinetics.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 03 Alkanes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 02 Structure and Properties.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 01 Introduction.pdf
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 27 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 26 Orbitals and Organic Chemistry - Pericyclic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 25 Secondary Metabolites - An Introduction to Natural Products Chemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 24 Biomolecules - Nucleic Acids and Their Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 23 Biomolecules - Lipids and Their Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 22 Carbohydrate Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 21 Biomolecules - Carbohydrates.ppt
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 15 Conjugated Systems.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 16 Aromatic Compounds.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 17 Reactions of Aromatic Compounds.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 18 Ketones and Aldehydes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 19 Amines.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 20 Carboxylic Acids.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 21 Carboxylic acid Derivatives.pdf
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)CHM 201 Introduction and Review - Structure and Bonding.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Structure and Bonding of Organic Molecules.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alcohols-structure and synthesis 2.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Overview.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkyl Halides from Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkynes McMurry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Benzene and Aromaticity(2011).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acids Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Chemistry of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conformational Analysis.ppt
