《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conformational Analysis

Conformational Analysis Newman Projections Ring Strain Cyclohexane Conformations
Conformational Analysis Newman Projections Ring Strain Cyclohexane Conformations

Views of Ethane 1.10A H-109.6°、/ H 1C H 154A H H ethane ethane ethane
Views of Ethane
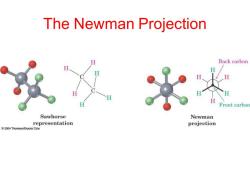
The Newman Projection H Back carbon Front carbon Sawhorse Newman representation projection 2004 Thomson/Brooks Cole
The Newman Projection
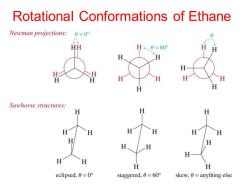
Rotational Conformations of Ethane Newman projections: 0=0° HH H、0=60 H H H H H H H H H Sawhorse structures: H H H H H H H H H H eclipsed,0=0° staggered,0=60° skew,0=anything else
Rotational Conformations of Ethane

Definitions Conformations-Different spatial arrangments that a molecule can adopt due to rotation about sigma bonds. ● Staggered-A low energy conformation where the bonds on adjacent atoms bisect each other(600 dihedral angle), maximizing the separation. Eclipsed-A high energy conformation ● where the bonds on adjacent atoms are aligned with each other(0 dihedral angle)
Definitions • Conformations - Different spatial arrangments that a molecule can adopt due to rotation about sigma bonds. • Staggered - A low energy conformation where the bonds on adjacent atoms bisect each other (60o dihedral angle), maximizing the separation. • Eclipsed - A high energy conformation where the bonds on adjacent atoms are aligned with each other (0o dihedral angle)
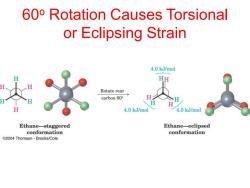
60o Rotation Causes Torsional or Eclipsing Strain 4.0 kJ/mol HH Rotate rear carbon60° 4.0 kJ/mol 4.0 k.J/mol Ethane-staggered Ethane-eclipsed conformation conformation C2004 Thomson-Brooks/Cole
60o Rotation Causes Torsional or Eclipsing Strain

Types of Strain Steric -Destabilization due to the repulsion between the electron clouds of atoms or groups. Groups try to occupy some common space. Torsional Destabilization due to the repulsion between pairs of bonds caused by the electrostatic repulsion of the electrons in the bonds.Groups are eclipsed. Angle Destabilisation due to distortion of a bond angle from it's optimum value caused by the electrostatic repulsion of the electrons in the bonds.e.g.cyclopropane
Types of Strain • Steric - Destabilization due to the repulsion between the electron clouds of atoms or groups. Groups try to occupy some common space. • Torsional - Destabilization due to the repulsion between pairs of bonds caused by the electrostatic repulsion of the electrons in the bonds. Groups are eclipsed. • Angle - Destabilisation due to distortion of a bond angle from it's optimum value caused by the electrostatic repulsion of the electrons in the bonds. e.g. cyclopropane

Definitions Anti Description given to two substitutents attached to adjacent atoms when their bonds are at 180 with respect to each other. Syn Description given to two substitutents attached to adjacent atoms when their bonds are at 0o with respect to each other. Gauche -Description given to two substitutents attached to adjacent atoms when their bonds are at 600 with respect to each other
Definitions • Anti - Description given to two substitutents attached to adjacent atoms when their bonds are at 180o with respect to each other. • Syn - Description given to two substitutents attached to adjacent atoms when their bonds are at 0o with respect to each other. • Gauche - Description given to two substitutents attached to adjacent atoms when their bonds are at 60o with respect to each other
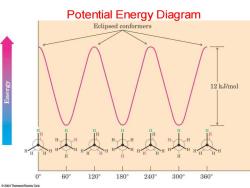
Potential Energy Diagram Eclipsed conformers siau出 12 kJ/mol 0° 60° 120° 180 240° 300° 360 2004 Thomson/Brooks Cole
Potential Energy Diagram
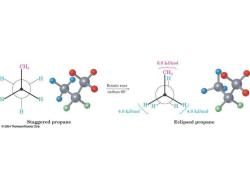
6.0 k.J/mol CHs CH H Rotate rear carbon 60 4.0 k.J/mol 4.0 k.J/mol Staggered propane Eclipsed propane 2004 Thomson/Brooks Cole
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Chemistry of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acids Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Benzene and Aromaticity(2011).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkynes McMurry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkyl Halides from Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Overview.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alcohols-structure and synthesis 2.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Structure and Bonding of Organic Molecules.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)CHM 201 Introduction and Review - Structure and Bonding.pptx
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 21 Carboxylic acid Derivatives.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 20 Carboxylic Acids.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 19 Amines.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 18 Ketones and Aldehydes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 17 Reactions of Aromatic Compounds.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 16 Aromatic Compounds.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 15 Conjugated Systems.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 14 Ethers and Epoxides.pdf
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conjugated Dienes and U.V. Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Enols and Enolates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ethers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Functional Groups.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared(IR)spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Nomenclature of Saturated Hydrocarbons.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Proton NMR Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared Spectroscopy and Mass Spectrometry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 01 Structure And Bonding(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 02 Alkanes and cycloalkane.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 03 functional groups.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 04 stereochemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 05 Nucleophiles and Electrophiles.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 06 reactions and mechanisms.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 07 Acids and Bases.pdf
