《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Mass Spectrometry
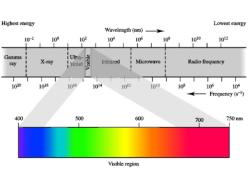
Highest energy Lowest energy Wavelength (nm) 10-2 10P 102 10 10的 108 1010 102 Gamma i Ulra- ray X-ray iviolet Inirared Microwave Radio frequency 1020 1018 106 104 102 1010 109 10的 104 Frequency (s-1) 400 500 600 700 750nm Visible region
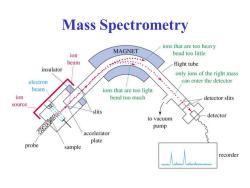
Mass Spectrometry ions that are too heavy MAGNET ion bend too little beam flight tube insulator only ions of the right mass electron can enter the detector beam ions that are too light ion bend too much detector slits source slits detector to vacuum pump accelerator plate probe sample recorder
Mass Spectrometry
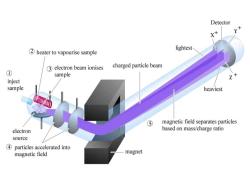
Detector lightest 2 heater to vapourise sample 3 electron beam ionises charged particle beam ① sample Z inject sample heaviest ⑤ magnetic field separates particles electron based on mass/charge ratio source 4 particles accelerated into magnetic field magnet
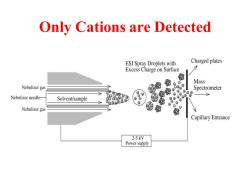
Only Cations are Detected ESI Spray Droplets with Charged plates Excess Charge on Surface 倒 Mass Nebulizer gas ⊕ Spectrometer ⊕© Nebulizer needle- Solvent/sample 8o88 Nebulizer gas 我 Capillary Entrance 2-5kV Power supply
Only Cations are Detected
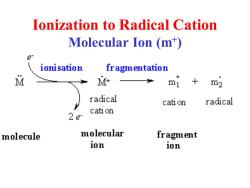
lonization to Radical Cation Molecular Ion (m) ionisation fragmentation M M+ mI m2 radical cati on radical cati on 22 molecule molecular fragment ion ion
Ionization to Radical Cation Molecular Ion (m+ )

Glossary Molecular ion -The ion obtained by the loss of one electron from the molecule (m) Base peak-The most intense peak in the MS, assigned 100%intensity Radical cation-positively charged species with an odd number of electrons Fragment ions-Lighter cations (and radical cations)formed by the decomposition of the molecular ion.These often correspond to stable carbcations. .m/z-mass to charge ratio
Glossary • Molecular ion - The ion obtained by the loss of one electron from the molecule (m+ ) • Base peak - The most intense peak in the MS, assigned 100% intensity • Radical cation - positively charged species with an odd number of electrons • Fragment ions - Lighter cations (and radical cations) formed by the decomposition of the molecular ion. These often correspond to stable carbcations. • m/z - mass to charge ratio
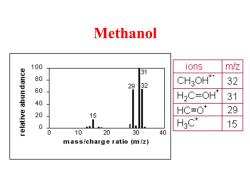
Methanol 100 ions m/z 31 aouepunge 80 29 32 CH3OH* 32 60 H2C=0H* 31 40 20 HC≡O 29 15 0 H3C* 15 0 10 20 30 40 mass/charge ratio (m/z)
Methanol
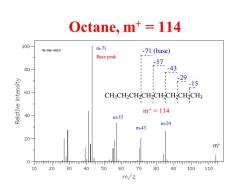
Octane,m+114 100 1s-N1-0560 m-71 -71(base) Base peak - 57 80 -43 -29 -15 60 CH:CH2CH-CH-CH-CH-CH2CH3 m+=114 40 m-57 m-29 m-43 20 m+ 0 -rrmmmllmrHrmhhpmtmmhmmmmpmr 10 20 30 40 50 60 70 80 90 100 110 m/z
Octane, m+ = 114 CH3CH2CH2CH2CH2CH2CH2CH3 m+ = 114 -15 -29 -43 -57 -71 (base) m-29 m-43 m-57 m-71 Base peak m+
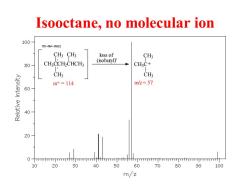
Isooctane,no molecular ion 100 M3-NW-0662 CH3CH3 loss of CH3 CH:CCH2CHCH3 (isobutyl) 80 CH3C+ CHs CH3 m+=114 m/z=57 60 BAlDley 40 20 10 20 30 40 50 60 70 80 90 100 m/z
Isooctane, no molecular ion CH3CCH2CHCH3 CH3 CH3 CH3 m+ = 114 loss of (isobutyl). CH3C CH3 CH3 + m/z = 57
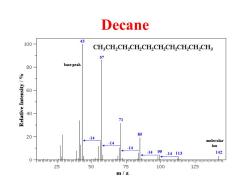
Decane 43 100- CH.CH.CH.CH.CH.CH.CH.CH.CHCH 57 80 base peak 3 HSUu 60 SAREPH 40 71 85 20 -14 -14 molecular -14 ion 149914113 142 0-mmmtmirmmmmimmtnmmmhmmmjrmmmnmmmmjmmmmmmmim 25 50 75 100 125 m/z
Decane
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared(IR)spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Functional Groups.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ethers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Enols and Enolates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conjugated Dienes and U.V. Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conformational Analysis.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Chemistry of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acids Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Benzene and Aromaticity(2011).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkynes McMurry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkyl Halides from Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Overview.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alcohols-structure and synthesis 2.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Structure and Bonding of Organic Molecules.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)CHM 201 Introduction and Review - Structure and Bonding.pptx
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 21 Carboxylic acid Derivatives.pdf
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Nomenclature of Saturated Hydrocarbons.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Proton NMR Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared Spectroscopy and Mass Spectrometry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 01 Structure And Bonding(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 02 Alkanes and cycloalkane.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 03 functional groups.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 04 stereochemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 05 Nucleophiles and Electrophiles.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 06 reactions and mechanisms.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 07 Acids and Bases.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 08 Alkenes and Alkynes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 09 Aromatic chemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 10 Aldehydes and ketones.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 11 Carboxylic acids and carboxylic acid derivatives.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 01 Introduction(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 02 Structure and Properties of OM.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 03 Brief Introduction and Nomenclature of OC.pdf
