《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Substitution and Elimination

Substitution and Elimination Reactions of Alkyl Halides
Substitution and Elimination Reactions of Alkyl Halides

Substitution,Nucleophilic, Bimolecular-S2 一人]人 transition state Rate k[Nuc:][R-X] Second Order Rate Kinetics
Substitution, Nucleophilic, Bimolecular – SN2 C X + − Nuc : Nuc C X Nuc C + X transition state − − Rate = k[Nuc: ][R-X] Second Order Rate Kinetics
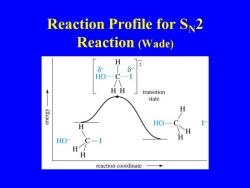
Reaction Profile for Sv2 Reaction (Wade) H 8 HO---C---I HH transition state H K.oua HO H reaction coordinate
Reaction Profile for SN2 Reaction (Wade)
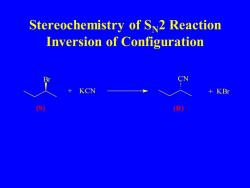
Stereochemistry of S2 Reaction Inversion of Configuration KCN KBr S R
Stereochemistry of SN2 Reaction Inversion of Configuration Br + KCN CN + KBr (S) (R)
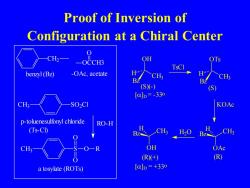
Proof of Inversion of Configuration at a Chiral Center CH2一 _OCCH3 TsCl benzyl (BZ) -OAc,acetate 6m.. CH CH; BZ (S(-) (S [a]D=-33o CH3- S02C1 KOAc p-toluenesulfonyl chloride RO-H (Ts-CI) BZ CH H20 CH3- -R OH OAc (R(+) R) a tosylate (ROTs) [a]D=+33o
Proof of Inversion of Configuration at a Chiral Center CH2 benzyl (Bz) CH SO2Cl 3 p-toluenesulfonyl chloride (Ts-Cl) CH3 S O O O R RO-H a tosylate (ROTs) OH CH3 Bz H []D = -33o (S)(-) TsCl OTs CH3 Bz H (S) KOAc OCCH3 O -OAc, acetate OAc Bz CH3 H (R) OH Bz CH3 H []D = +33o (R)(+) H2O
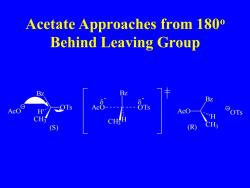
Acetate Approaches from 180 Behind Leaving Group AcO " OTs S (R
Acetate Approaches from 180o Behind Leaving Group OTs Bz CH3 AcO H Bz CH3 H AcO OTs - - AcO Bz CH3 H (S) (R) OTs
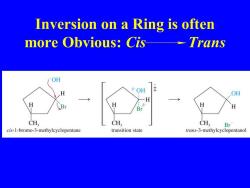
Inversion on a Ring is often more Obvious:Cis--Trans OH OH OH H H CH CH CH, Br cis-1-bromo-3-methylcyclopentane transition state trans-3-methylcyclopentanol
Inversion on a Ring is often more Obvious: Cis Trans
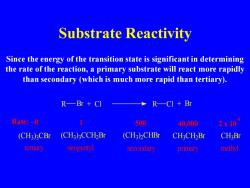
Substrate Reactivity Since the energy of the transition state is significant in determining the rate of the reaction,a primary substrate will react more rapidly than secondary (which is much more rapid than tertiary). R—Br+CI R一CI+Br Rate:~0 500 40,000 2x10 (CH3)3CBr (CH3)3CCH2Br (CH3)2CHBr CH3CH2Br CH3Br tertiary neopenty secondary primary methyl
Substrate Reactivity Since the energy of the transition state is significant in determining the rate of the reaction, a primary substrate will react more rapidly than secondary (which is much more rapid than tertiary). 6 tertiary neopentyl secondary primary methyl Rate: ~0 (CH3 ) 3 CBr C H C H3 Br (CH3) 3CCH2Br (CH3) 2CHBr 3 C H2 Br R Br + Cl R Cl + Br 1 500 40,000 2 x 10
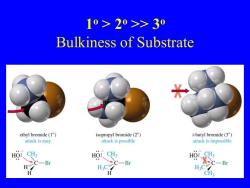
10>20>>30 Bulkiness of Substrate ethyl bromide(1) isopropyl bromide(2) t-butyl bromide(3) attack is easy attack is possible attack is impossible HO:、CH HO:、CH3 C一Br C-Br H H.C H.cC-Br H CH3
1 o > 2o >> 3o Bulkiness of Substrate

Polar,Aprotic Solvents Solvents should be able to "cage"the metal cation CH3SCH:CHCN HCN(CH3)CHCCH DMSO acetonitrile DMF acetone Polar,protic solvents lower energy of nucleophile HOCH3 by solvation CH3OH---Br---HOCH; CH3OH
Polar, Aprotic Solvents by solvation Polar, protic solvents lower energy of nucleophile C H3O H HOCH3 C H3O H Br HOCH3 acetone O C H3 C N C H3 CCH3 DMSO acetonitrile DMF O HCN(CH3 ) 2 O C H3 SCH3 Solvents should be able to "cage" the metal cation
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Proton NMR Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Nomenclature of Saturated Hydrocarbons.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared(IR)spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Functional Groups.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ethers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Enols and Enolates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conjugated Dienes and U.V. Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conformational Analysis.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Chemistry of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acids Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Benzene and Aromaticity(2011).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkynes McMurry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkyl Halides from Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared Spectroscopy and Mass Spectrometry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 01 Structure And Bonding(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 02 Alkanes and cycloalkane.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 03 functional groups.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 04 stereochemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 05 Nucleophiles and Electrophiles.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 06 reactions and mechanisms.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 07 Acids and Bases.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 08 Alkenes and Alkynes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 09 Aromatic chemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 10 Aldehydes and ketones.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 11 Carboxylic acids and carboxylic acid derivatives.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 01 Introduction(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 02 Structure and Properties of OM.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 03 Brief Introduction and Nomenclature of OC.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 04 Structure and Stereochemistry of Alkanes(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 05 The Study of Chemical Reactions(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 06 Stereochemistry(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 04 Structure and Stereochemistry of Alkanes.pdf
