西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 04 Structure and Stereochemistry of Alkanes

0 花华院 Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 4 Structure and Stereochemistry of Alkanes By Junru Wang Email:wangjr07@163.com
By Junru Wang Email: wangjr07@163.com Chapter 4 Structure and Stereochemistry of Alkanes Organic Chemistry, 6th Edition L. G. Wade, Jr

0环4 Key Notes 花率院 ■Mechanisms ■Conformation ■Ring strain ■Conformers ■Cis-trans isomers
Key Notes Mechanisms Conformation Ring strain Conformers Cis-trans isomers

0 CONTENTS 发牌院 Classification of Hydrocarbons Physical properties Reactions Mechanisms of Alkanes Structure Conformation of Alkanes Conformation of Cycloalkanes
CONTENTS Classification of Hydrocarbons Physical properties Reactions & Mechanisms of Alkanes Structure & Conformation of Alkanes Conformation of Cycloalkanes
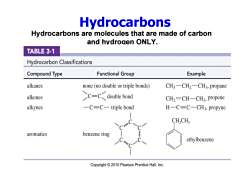
Hydrocarbons Hydrocarbons are molecules that are made of carbon and hvdrogen ONLY. TABLE 3-1 Hydrocarbon Classifications Compound Type Functional Group Example alkanes none(no double or triple bonds) CH3-CH2-CH3,propane alkenes CCdouble bond CH2=CH-CH3,propene alkynes -C=C-triple bond H-C=C-CH3,propyne CH,CH aromatics benzene ring ethylbenzene Copyright2010 Pearson Prentice Hall,Inc
Hydrocarbons are molecules that are made of carbon and hydrogen ONLY. Hydrocarbons

Common AlkyI Groups One carbon Two carbons Three carbons CH3 CH3一 CH3-CH2- CH3-CH2-CH2- CH3-CH- methyl group ethyl group propyl group isopropyl group (or“-propyl group") Four carbons CH3 CH3 CH3 CH3-CH2-CH2-CH2- CH3-CH-CH2一 CH3-CH2-CH- CH3-C- butyl group isobutyl group sec-butyl group (or“n-butyl group") CH3 tert-butyl group (or“t-butyl group") Copyright 2010 Pearson Prentice Hall,Inc
Common Alkyl Groups
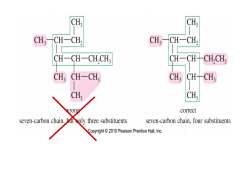
CH CH CH: CH-CH, CH-CH, CH-CH-CH,CH; CH-CH-CH,CH, CH CH一CH, CH: CH-CH CH; CH; correct seven-carbon chain,bt ly three substituents seven-carbon chain,four substituents Copyright2010 Pearson Prentice Hall,Inc
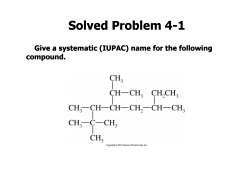
Solved Problem 4-1 Give a systematic (IUPAC)name for the following compound. CH CH-CH, CH,CH CH,一CH-CH一CH,-CH-CH CH,C一CH CH; Copyright2010 Pearsan Prentice Hall Ins
Solved Problem 4-1 Give a systematic (IUPAC) name for the following compound

Solved Problem 4-1:Solution The longest carbon chain contains eight carbon atoms,so this compound is named as an octane.Numbering from left to right gives the first branch on C2;numbering from right to left gives the first branch on C3,so we number from left to right. CH 8 CH-CH, CH,CH CH, CH-4CH-CH,-CH-CH CH CH: CH ce Hall,Inc. 4-isopropyl-2,2,3,6-tetramethyloctane
The longest carbon chain contains eight carbon atoms, so this compound is named as an octane. Numbering from left to right gives the first branch on C2; numbering from right to left gives the first branch on C3, so we number from left to right. Solved Problem 4-1: Solution 4-isopropyl-2,2,3,6-tetramethyloctane

0不年 SEC 1 Physical Properties Solubility:hydrophobic Density:less than 1 g/mL Boiling points increase with increasing carbons (little less for branched chains). Melting points increase with increasing carbons(less for odd-number of carbons)
SEC 1 Physical Properties Solubility: hydrophobic Density: less than 1 g/mL Boiling points increase with increasing carbons (little less for branched chains). Melting points increase with increasing carbons (less for odd-number of carbons)
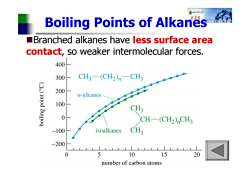
0t女车 Boiling Points of Alkanes Branched alkanes have less surface area contact,so weaker intermolecular forces. 400 300 CH3一(CH2)m-CH 200 uod n-alkanes 100 CH3 3um!oq 0 CH-(CH2)CH3 -100 isoalkanes CH3 -200 0 5 10 15 20 number of carbon atoms
Boiling Points of Alkanes Branched alkanes have less surface area contact, so weaker intermolecular forces
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 06 Stereochemistry(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 05 The Study of Chemical Reactions(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 04 Structure and Stereochemistry of Alkanes(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 03 Brief Introduction and Nomenclature of OC.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 02 Structure and Properties of OM.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 01 Introduction(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 11 Carboxylic acids and carboxylic acid derivatives.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 10 Aldehydes and ketones.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 09 Aromatic chemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 08 Alkenes and Alkynes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 07 Acids and Bases.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 06 reactions and mechanisms.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 05 Nucleophiles and Electrophiles.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 04 stereochemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 03 functional groups.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 02 Alkanes and cycloalkane.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 01 Structure And Bonding(主讲:王俊儒).pdf
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Substitution and Elimination.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 17 & 18 Aromatic chemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 05 The Study of Chemical Reactions.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 06 Stereochemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 08 & 09 Alkenes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 19 Ketones and Aldehydes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 20 Amines.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 21 Carboxylic Acids.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 01 Introduction and Review(主讲:王俊儒).ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 14 & 15 Alcohols.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 17 & 18 Aromatic chemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 20 Amines.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 02 Structure and Properties of OMs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 03 Brief Introduction and Nomenclature of OCs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 04 Structure and Stereochemistry of Alkanes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 05 The Study of Chemical Reactions.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 06 Stereochemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 08 & 09 Alkenes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 10 Alkynes.ppt
