西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 17 & 18 Aromatic chemistry

Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 17 e 18 Aromatic chemistry Key Notes Aromaticity;Resonance Structure;Huckelrule; Electrophilic substitutions;Deactivating group; By Junru Wang Email:wangjr07@163.com 西北农林科技大学理学院
Chapter 17 & 18 Aromatic chemistry Organic Chemistry, 6th Edition L. G. Wade, Jr. By Junru Wang Email: wangjr07@163.com 西北农林科技大学理学院 Key Notes Aromaticity; Resonance Structure; Hückel rule; Electrophilic substitutions; Deactivating group;

CONTENT Structure Physical Properties Aromaticity reactivity Hiickel rule Preparation of Ar compounds Electrophilic substitutions Effects of substituents on E.S. -Synthesis of mono-,di-and tri-substituted benzenes Other reactions -Oxidation and reduction -Nucleophilic Substitution
CONTENT ❖Structure & Physical Properties ❖Aromaticity & reactivity ◼Hückel rule ❖Preparation of Ar compounds ❖Electrophilic substitutions ❖Effects of substituents on E.S. ◼Synthesis of mono-, di- and tri-substituted benzenes ❖Other reactions ◼Oxidation and reduction ◼Nucleophilic Substitution

Sec 1 Structure Physical Properties Discovery of Benzene -Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. -Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to be C6H6. -Other related compounds with low C:H ratios had a pleasant smell,so they were classified as aromatic
Sec 1 Structure & Physical Properties ❖Discovery of Benzene ◼Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. ◼Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to be C6H6 . ◼Other related compounds with low C:H ratios had a pleasant smell, so they were classified as aromatic
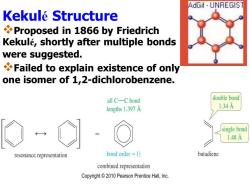
AdGif-UNREGIST Kekule Structure Proposed in 1866 by Friedrich Kekule,shortly after multiple bonds were suggested. Failed to explain existence of only one isomer of 1,2-dichlorobenzene. all C-C bond double bond lengths 1.397 A 1.34A single bond 1.48A resonance representation bond order=1 butadiene combined representation Copyright2010 Pearson Prentice Hall,Inc
KekuléStructure ❖Proposed in 1866 by Friedrich Kekulé, shortly after multiple bonds were suggested. ❖Failed to explain existence of only one isomer of 1,2-dichlorobenzene
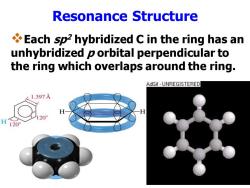
Resonance Structure Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring. AdGif UNREGISTERED 1.397 H120°
Resonance Structure ❖Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring
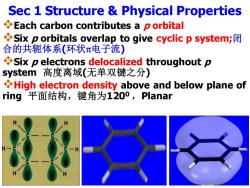
Sec 1 Structure Physical Properties Each carbon contributes a p orbital Six p orbitals overlap to give cyclic p system; 合的共轭体系(环状π电子流) Six p electrons delocalized throughout p system高度离域(无单双键之分) High electron density above and below plane of ring平面结构,键角为1200,Planar
Sec 1 Structure & Physical Properties ❖Each carbon contributes a p orbital ❖Six p orbitals overlap to give cyclic p system;闭 合的共轭体系(环状电子流) ❖Six p electrons delocalized throughout p system 高度离域(无单双键之分) ❖High electron density above and below plane of ring 平面结构,键角为1200 ,Planar
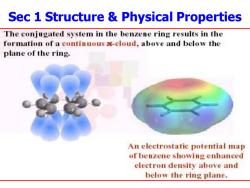
Sec 1 Structure Physical Properties The conjugated system in the benzene ring results in the formation of a continuous -cloud,above and below the plane of the ring. An electrostatic potential map of benzene showing enhanced electron density above and below the ring plane
Sec 1 Structure & Physical Properties
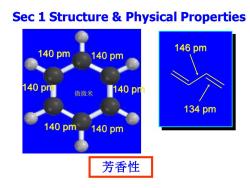
Sec 1 Structure Physical Properties 140pm 146pm 140pm 140pm 微微米 140p 134pm 140pm 140pm 芳香性
Sec 1 Structure & Physical Properties 芳香性 140 pm 140 pm 140 pm 140 pm 140 pm 140 pm 146 pm 134 pm 微微米
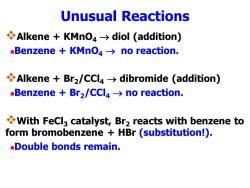
Unusual Reactions Alkene KMno>diol (addition) -Benzene KMnO4->no reaction. Alkene Br2/CCl4>dibromide (addition) -Benzene Br2/CCl4->no reaction. With FeCl3 catalyst,Br2 reacts with benzene to form bromobenzene HBr (substitution!). -Double bonds remain
Unusual Reactions ❖Alkene + KMnO4 → diol (addition) ◼Benzene + KMnO4 → no reaction. ❖Alkene + Br2/CCl4 → dibromide (addition) ◼Benzene + Br2/CCl4 → no reaction. ❖With FeCl3 catalyst, Br2 reacts with benzene to form bromobenzene + HBr (substitution!). ◼Double bonds remain
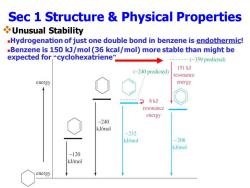
Sec 1 Structure Physical Properties Unusual Stability -Hydrogenation of just one double bond in benzene is endothermic! -Benzene is 150 kJ/mol(36 kcal/mol)more stable than might be expected for,“cyclohexatriene” (-359 predicted) 151kJ (-240 predicted) resonance energy energy 8kJ resonance energy -240 kJ/mol -232 kJ/mol -208 kJ/mol -120 kJ/mol energy
Sec 1 Structure & Physical Properties ❖Unusual Stability ◼Hydrogenation of just one double bond in benzene is endothermic! ◼Benzene is 150 kJ/mol (36 kcal/mol) more stable than might be expected for “cyclohexatriene
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 14 & 15 Alcohols.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 01 Introduction and Review(主讲:王俊儒).ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 21 Carboxylic Acids.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 20 Amines.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 19 Ketones and Aldehydes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 08 & 09 Alkenes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 06 Stereochemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 05 The Study of Chemical Reactions.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 17 & 18 Aromatic chemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 04 Structure and Stereochemistry of Alkanes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 06 Stereochemistry(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 05 The Study of Chemical Reactions(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 04 Structure and Stereochemistry of Alkanes(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 03 Brief Introduction and Nomenclature of OC.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 02 Structure and Properties of OM.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 01 Introduction(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 11 Carboxylic acids and carboxylic acid derivatives.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 10 Aldehydes and ketones.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 20 Amines.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 02 Structure and Properties of OMs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 03 Brief Introduction and Nomenclature of OCs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 04 Structure and Stereochemistry of Alkanes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 05 The Study of Chemical Reactions.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 06 Stereochemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 08 & 09 Alkenes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 10 Alkynes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 19 Ketones and Aldehydes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 21 Carboxylic Acids.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 22 Carboxylic Acid Derivatives.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 23 Condensation and Alpha Substitution of Carbonyl Compounds.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 24 Carbohydrates and Nucleic Acids.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 25 Amino Acids, Peptides and Proteins.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 26 Lipids.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 02 Structure and Properties of OMs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 14 & 15 Alcohols.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 19 Ketones and Aldehydes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 20 Amines.ppt
