西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 23 Condensation and Alpha Substitution of Carbonyl Compounds

Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 23 Condensation and Alpha Substitution of Carbonyl Compounds
Chapter 23 Condensation and Alpha Substitution of Carbonyl Compounds Organic Chemistry, 6th Edition L. G. Wade, Jr
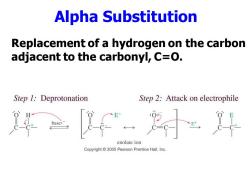
Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,C=O. Step 1:Deprotonation Step 2:Attack on electrophile base: enolate ion Copyright 2005 Pearson Prentice Hall,Inc
Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl, C=O
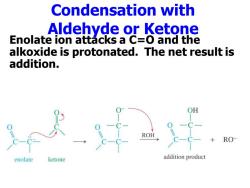
Condensation with Aldehyde or Ketone Enolate ion attacks a C=O and the alkoxide is protonated.The net result is addition. RO enolate ketone addition product
Condensation with Aldehyde or Ketone Enolate ion attacks a C=O and the alkoxide is protonated. The net result is addition
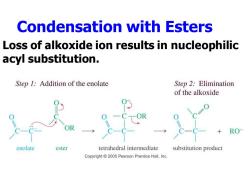
Condensation with Esters Loss of alkoxide ion results in nucleophilic acyl substitution. Step 1:Addition of the enolate Step 2:Elimination of the alkoxide RO enolate ester tetrahedral intermediate substitution product Copyright 2005 Pearson Prentice Hall,Inc
Condensation with Esters Loss of alkoxide ion results in nucleophilic acyl substitution
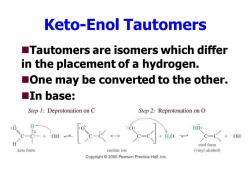
Keto-Enol Tautomers Tautomers are isomers which differ in the placement of a hydrogen. One may be converted to the other. ■In base: Step 1:Deprotonation on C Step 2:Reprotonation on O HO: DH +-OH enol form keto form enolate ion (vinyl alcohol) Copyright 2005 Pearson Prentice Hall,Inc
Keto-Enol Tautomers ◼Tautomers are isomers which differ in the placement of a hydrogen. ◼One may be converted to the other. ◼In base:
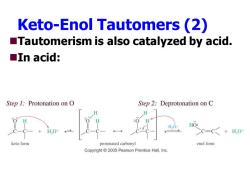
Keto-Enol Tautomers(2) Tautomerism is also catalyzed by acid. ■In acid: Step 1:Protonation on O Step 2:Deprotonation on C H H,0: HO: H,O+ C=C+H,0 keto form protonated carbonyl enol form Copyright 2005 Pearson Prentice Hall,Inc
Keto-Enol Tautomers (2) ◼Tautomerism is also catalyzed by acid. ◼In acid:

Equilibrium Amounts For aldehydes and ketones,the keto form is greatly favored at equilibrium. An enantiomer with an enolizable enolizable hydrogens CH: CH CH, H+orOH -a carbons (R)configuration enol (achiral) (S)configuration Copyright 2005 Pearson Prentice Hall,Inc
Equilibrium Amounts ◼For aldehydes and ketones, the keto form is greatly favored at equilibrium. ◼An enantiomer with an enolizable hydrogen can form a racemic mixture

Acidity of a-Hydrogens p for a-H of aldehyde or ketone ~20. Much more acidic than alkane or alkene(pK>40)or alkyne (pK =25). Less acidic than water (p=15.7)or alcohol (p 16-19). In the presence of hydroxide or alkoxide ions,only a small amount of enolate ion is present at equilibrium
Acidity of -Hydrogens ◼pKa for -H of aldehyde or ketone ~20. ◼Much more acidic than alkane or alkene (pKa > 40) or alkyne (pKa = 25). ◼Less acidic than water (pKa = 15.7) or alcohol (pKa = 16-19). ◼In the presence of hydroxide or alkoxide ions, only a small amount of enolate ion is present at equilibrium
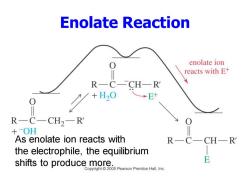
Enolate Reaction enolate ion reacts with E+ R—C-CH—R →E R一C一CH2一R +OH As enolate ion reacts with R一 the electrophile,the equilibrium shifts to produce more. Copyright 2005 Pearson Prentice Hall,Inc
Enolate Reaction As enolate ion reacts with the electrophile, the equilibrium shifts to produce more

Acid-Base Reaction to Form Enolate Very strong base is required for complete reaction.Example: CH3 CH3 CH3-CH CH3-CH N一H CaH Li C.Hio N: Li计 CH-CH n-butyllithium butane CH3一CH CH, CH; diisopropylamine lithium diisopropylamide (LDA) Copyright 2005 Pearson Prentice Hall,Inc. (i-CH)2N-Li+ (i-C2H)2N-H cyclohexanone LDA lithium enolate (pK,=36 (pK.=19) of cyclohexanone (100%) Copyright 2005 Pearson Prentice Hall,Inc
Acid-Base Reaction to Form Enolate Very strong base is required for complete reaction. Example:
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 22 Carboxylic Acid Derivatives.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 21 Carboxylic Acids.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 19 Ketones and Aldehydes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 10 Alkynes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 08 & 09 Alkenes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 06 Stereochemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 05 The Study of Chemical Reactions.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 04 Structure and Stereochemistry of Alkanes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 03 Brief Introduction and Nomenclature of OCs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 02 Structure and Properties of OMs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 20 Amines.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 17 & 18 Aromatic chemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 14 & 15 Alcohols.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 01 Introduction and Review(主讲:王俊儒).ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 21 Carboxylic Acids.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 20 Amines.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 19 Ketones and Aldehydes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 08 & 09 Alkenes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 24 Carbohydrates and Nucleic Acids.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 25 Amino Acids, Peptides and Proteins.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 26 Lipids.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 02 Structure and Properties of OMs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 14 & 15 Alcohols.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 19 Ketones and Aldehydes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 20 Amines.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 23 Condensation and Alpha Substitution of Carbonyl Compounds.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 01 Introduction and Preview(主讲:王俊儒).ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 03 Brief Introduction and Nomenclature of OCs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 04 Structure and Stereochemistry of Alkanes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 05 The Study of Chemical Reactions.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 06 Stereochemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 08 & 09 Alkenes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 10 Alkynes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 17 & 18 Aromatic chemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 21 & 22 Carboxylic Acids & Derivatives.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 01 Structure and Bonding.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 00 Introduction to the course.ppt
