西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 10 Alkynes

有机化学oRGAnIc©EmISTRY 盖讲:王德儒87092829(0) 理学院寇化素理科接2层206 Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 10 AlRynes Key Notes Electronic Structure;Acidity;Terminal alkynes Homework.10-22:10-23:(P274)
Chapter 10 Alkynes Organic Chemistry, 6th Edition L. G. Wade, Jr. Key Notes Electronic Structure; Acidity; Terminal alkynes 有机化学ORGANIC CHEMISTRY 主讲:王俊儒 87092829(O) 理学院应化系理科楼2层C206 Homework:10-22; 10-23; (P274)

音秋标特大对 CONTENTS Properties Preparation ■Reactions of alkynes Electrophilic additions to alkynes Reduction Oxidation of alkynes OSpecial reactions of terminal alkynes
CONTENTS ◼Properties ◼Preparation ◼Reactions of alkynes ⚫Electrophilic additions to alkynes ⚫Reduction & Oxidation of alkynes ⚫Special reactions of terminal alkynes
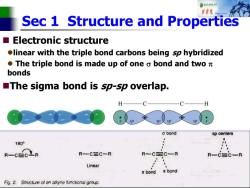
自秋转达对 报中院 Sec 1 Structure and Properties Electronic structure olinear with the triple bond carbons being sp hybridized ●The triple bond is made up of one o bond and twoπ bonds The sigma bond is sp-sp overlap. H a bond sp centers 1800 R-CECLR R-C三C~R Linear Fig.2.Structure of an akyne functional group
Sec 1 Structure and Properties ◼ Electronic structure ⚫linear with the triple bond carbons being sp hybridized ⚫ The triple bond is made up of one bond and two bonds ◼The sigma bond is sp-sp overlap
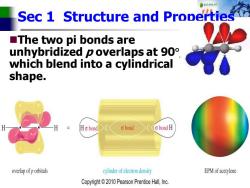
Sec 1 S Structure and Properties ■The two pi bonds are unhybridized p overlaps at 90 which blend into a cylindrical shape. Ho bond 6bond o bond H overlap of p orbitals cylinder of electron density EPM of acetylene Copyright2010 Pearson Prentice Hall,Inc
Sec 1 Structure and Properties ◼The two pi bonds are unhybridized p overlaps at 90 , which blend into a cylindrical shape
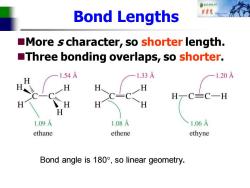
自秋不转大对 Bond Lengths More s character,so shorter length. Three bonding overlaps,so shorter. 1.54A 1.33 1.20A H H H H一CC一H H H H H 1.09A 1.08A 1.06A ethane ethene ethyne Bond angle is180°,so linear geometry
Bond Lengths ◼More s character, so shorter length. ◼Three bonding overlaps, so shorter. Bond angle is 180, so linear geometry

0标4化对 Acetylene Acetylene is used in welding torches. In pure oxygen,temperature of flame reaches 2800C. It would violently decompose to its elements,but the cylinder on the torch contains crushed firebricki耐火砖粉末wet with acetone to moderate it
Acetylene ◼Acetylene is used in welding torches. ◼In pure oxygen, temperature of flame reaches 2800C. ◼It would violently decompose to its elements, but the cylinder on the torch contains crushed firebrick耐火砖粉末wet with acetone to moderate it

自秋标特试 Sec 1 Structure and Properties Nonpolar,insoluble in water. Soluble in most organic solvents. Boiling points similar to alkane of same size. Less dense than water. Up to 4 carbons,gas at R.T
Sec 1 Structure and Properties ◼Nonpolar, insoluble in water. ◼Soluble in most organic solvents. ◼Boiling points similar to alkane of same size. ◼Less dense than water. ◼Up to 4 carbons, gas at R.T

自秋特大材 Acidity of Alkynes Terminal alkynes,R-C=C-H,are more acidic than other hydrocarbons. Acetylene -acetylide by NH2,but not by OH-or RO-. More s character,so pair of electrons in anion is held more closely to the nucleus. Less charge separation,so more stable
Acidity of Alkynes ◼Terminal alkynes, R-CC-H, are more acidic than other hydrocarbons. ◼Acetylene → acetylide by NH2 - , but not by OH- or RO- . ◼More s character, so pair of electrons in anion is held more closely to the nucleus. Less charge separation, so more stable
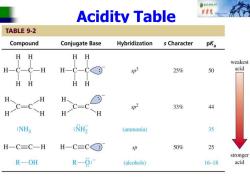
自秋不转大对 Acidity Table 中院 TABLE 9-2 Compound Conjugate Base Hybridization s Character pKa HH HH weakest H-C-C-H H-C-C© D3 25% 50 acid HH HH H、 H C=C H H p2 33% 44 :NH2 (ammonia) 35 H一C三C一H H一C=C© p 50% 25 stronger R一OH R-0: (alcohols) 16-18 acid
Acidity Table
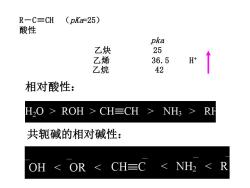
R一C=CH (pKa-25) 酸性 pka 乙炔 25 乙烯 36.5 H' 乙烷 42 相对酸性: H20 >ROH>CH≡CH > NH> 共轭碱的相对碱性: OH <OR<CH≡C<NH2<R
R-C≡CH (pKa=25) 酸性 pka 乙炔 25 乙烯 36.5 H+ 乙烷 42 相对酸性: 共轭碱的相对碱性:
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 08 & 09 Alkenes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 06 Stereochemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 05 The Study of Chemical Reactions.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 04 Structure and Stereochemistry of Alkanes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 03 Brief Introduction and Nomenclature of OCs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 01 Introduction and Preview(主讲:王俊儒).ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 23 Condensation and Alpha Substitution of Carbonyl Compounds.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 20 Amines.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 19 Ketones and Aldehydes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 14 & 15 Alcohols.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 02 Structure and Properties of OMs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 26 Lipids.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 25 Amino Acids, Peptides and Proteins.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 24 Carbohydrates and Nucleic Acids.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 23 Condensation and Alpha Substitution of Carbonyl Compounds.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 22 Carboxylic Acid Derivatives.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 21 Carboxylic Acids.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 19 Ketones and Aldehydes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 10 Alkynes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 17 & 18 Aromatic chemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 21 & 22 Carboxylic Acids & Derivatives.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 01 Structure and Bonding.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 00 Introduction to the course.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 02-Sp Brief Introduction and Nomenclature of OCs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 03 Organic compounds alkanes and their stereochemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 04 Organic compounds cycloalkanes and their stereochemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 05 Stereochemistry at Tetrahedral Centers.pptx
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 06 an overview of organic Reactions.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 09 aromatic compounds.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 12 Organohalides Nucleophilic Substitutions and Eliminations.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2018)McMurry OCB 3Ed Chapter 00 Introduction to the course(主讲:王俊儒).ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2019)McMurry OCB 3Ed Chapter 00 Introduction to the course.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2019)McMurry OCB 3Ed Chapter 01 Structure and Bonding(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2019)McMurry OCB 3Ed Chapter 02 Polar covalent Bonds acids and Bases.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2019)McMurry OCB 3Ed Chapter 02-Sp Brief Introduction and Nomenclature of OCs.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2019)McMurry OCB 3Ed Chapter 07 & 08 alkenes and alkynes.pdf
- 运城学院:《环境化学》课程教学资源(教案讲义)理论教学大纲 Environmental Chemistry.pdf
- 运城学院:《环境化学》课程教学资源(教案讲义)实验二 土壤对铜离子的吸附.pdf
- 运城学院:《环境化学》课程教学资源(教案讲义)第一章 绪论 Environmental Chemistry.pdf
