西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 21 & 22 Carboxylic Acids & Derivatives

有机化学ORGAnI©CHEMISTRY 主讲:主俊儒87092829(0) 理学院应化系理科接2层C206 Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 21 &T 22 Carboxylic Acids Derivatives Key Notes Hydrogen bonding;Acidity;nucleophilic substituetion claisen condensation;reduction, Homework:P732:21-18;21-19(f,g,h)P762:22-19(a-f)
Chapter 21 & 22 Carboxylic Acids & Derivatives Organic Chemistry, 6th Edition L. G. Wade, Jr. Key Notes Hydrogen bonding ; Acidity; nucleophilic substituetion; claisen condensation; reduction; 有机化学ORGANIC CHEMISTRY 主讲:王俊儒 87092829(O) 理学院应化系理科楼2层C206 Homework: P732: 21-18; 21-19(f,g,h); P762: 22-19(a-f)

Blister beetles secrete cantharidin,a powerful vesicant.Crushing a blister beetle between the fingers results in severe blistering of the skin.When horses eat hay containing blister beetles,they often die from The vinegaroon(whip-tail scorpion) gastroenteritis and kidney failure CH expels a defensive spray consisting of 84%acetic acid,5%octanoic acid. caused by cantharidin poisoning. and 11%water.Octanoic acid acts as a wetting and spreading agent. 斑蝥素 CH3O cantharidin
斑蝥素

CONTENTS ■REVIEW:NAMING STRUCTURE AND PROPERTIES NUCLEOPHILIC SUBSTITUTION ■PREPARATIONS ■REACTIONS ■ENOLATE REACTIONS
CONTENTS ◼REVIEW:NAMING ◼STRUCTURE AND PROPERTIES ◼NUCLEOPHILIC SUBSTITUTION ◼PREPARATIONS ◼REACTIONS ◼ENOLATE REACTIONS
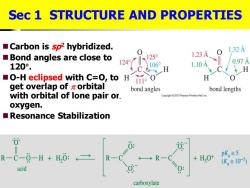
Sec 1 STRUCTURE AND PROPERTIES Carbon is sp2 hybridized. 1247X12 0 1.32A Bond angles are close to 1.23A 120°. 1060 1.10A 0.97 H O-H eclipsed with C=O,to H 111°0 H get overlap of zorbital bond angles bond lengths with orbital of lone pair or. Copyght2010 Pearson Prentice Hall Inc oxygen. Resonance Stabilization 0 R-C-0-H H,0: +H,0t pK,=5 R- (K≡10-5) acid carboxylate
◼Carbon is sp2 hybridized. ◼Bond angles are close to 120 . ◼O-H eclipsed with C=O, to get overlap of orbital with orbital of lone pair on oxygen. ◼Resonance Stabilization Sec 1 STRUCTURE AND PROPERTIES
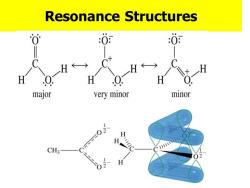
Resonance Structures :0 :0 YX H H 一H H Q major very minor minor H H CH3
Resonance Structures
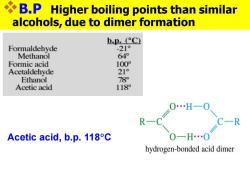
B.P Higher boiling points than similar alcohols,due to dimer formation bp.°C Formaldehyde -21° Methanol 64° Formic acid 100° Acetaldehyde 21° Ethanol 78 Acetic acid 118 0…H一0 R C-R Acetic acid,b.p.118C hydrogen-bonded acid dimer
❖B.P Higher boiling points than similar alcohols, due to dimer formation Acetic acid, b.p. 118C
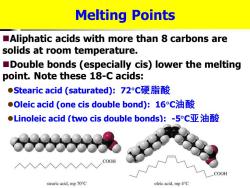
Melting Points Aliphatic acids with more than 8 carbons are solids at room temperature. Double bonds (especially cis)lower the melting point.Note these 18-C acids: ●Stearic acid(saturated):72c硬脂酸 ●Oleic acid(one cis double bond):16C油酸 ●Linoleic acid(two cis double bonds):-5c亚油酸 COOH COOH stearic acid.mp70°C oleic acid.mp 4C
Melting Points ◼Aliphatic acids with more than 8 carbons are solids at room temperature. ◼Double bonds (especially cis) lower the melting point. Note these 18-C acids: ⚫Stearic acid (saturated): 72C硬脂酸 ⚫Oleic acid (one cis double bond): 16C油酸 ⚫Linoleic acid (two cis double bonds): -5C亚油酸 硬脂酸,油酸,亚油酸

Solubility Water solubility decreases with the length of the carbon chain. Up to 4 carbons,acid is miscible in water. More soluble in alcohol. Also soluble in relatively nonpolar solvents like chloroform because it dissolves as a dimer
Solubility ◼Water solubility decreases with the length of the carbon chain. ◼Up to 4 carbons, acid is miscible in water. ◼More soluble in alcohol. ◼Also soluble in relatively nonpolar solvents like chloroform because it dissolves as a dimer
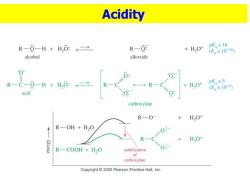
Acidity R-0-H+H,0:←→ R-0: pK。≡16 +HO+ (K。≡1016 alcohol alkoxide 0 R-C-0-H+H,:→ R HO+ pK≡5 + (K。=10-5) acid carboxylate R—O- +HO+ R-OH H2O R 0-0以 + HO+ R-COOH H2O stabilization 0 of carboxylate Copyright 2005 Pearson Prentice Hall,Inc
Acidity
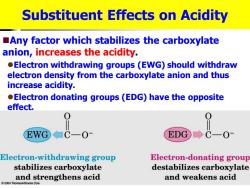
Substituent Effects on Acidity Any factor which stabilizes the carboxylate anion,increases the acidity. Electron withdrawing groups (EWG)should withdraw electron density from the carboxylate anion and thus increase acidity. Electron donating groups(EDG)have the opposite effect. EWG ■CO1 EDG C-0 Electron-withdrawing group Electron-donating group stabilizes carboxylate destabilizes carboxylate and strengthens acid and weakens acid 2004 Thomson/Brooks Cole
Substituent Effects on Acidity ◼Any factor which stabilizes the carboxylate anion, increases the acidity. ⚫Electron withdrawing groups (EWG) should withdraw electron density from the carboxylate anion and thus increase acidity. ⚫Electron donating groups (EDG) have the opposite effect
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 17 & 18 Aromatic chemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 10 Alkynes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 08 & 09 Alkenes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 06 Stereochemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 05 The Study of Chemical Reactions.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 04 Structure and Stereochemistry of Alkanes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 03 Brief Introduction and Nomenclature of OCs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 01 Introduction and Preview(主讲:王俊儒).ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 23 Condensation and Alpha Substitution of Carbonyl Compounds.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 20 Amines.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 19 Ketones and Aldehydes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 14 & 15 Alcohols.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 02 Structure and Properties of OMs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 26 Lipids.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 25 Amino Acids, Peptides and Proteins.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 24 Carbohydrates and Nucleic Acids.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 23 Condensation and Alpha Substitution of Carbonyl Compounds.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 22 Carboxylic Acid Derivatives.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 21 Carboxylic Acids.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 01 Structure and Bonding.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 00 Introduction to the course.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 02-Sp Brief Introduction and Nomenclature of OCs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 03 Organic compounds alkanes and their stereochemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 04 Organic compounds cycloalkanes and their stereochemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 05 Stereochemistry at Tetrahedral Centers.pptx
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 06 an overview of organic Reactions.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 09 aromatic compounds.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 12 Organohalides Nucleophilic Substitutions and Eliminations.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2018)McMurry OCB 3Ed Chapter 00 Introduction to the course(主讲:王俊儒).ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2019)McMurry OCB 3Ed Chapter 00 Introduction to the course.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2019)McMurry OCB 3Ed Chapter 01 Structure and Bonding(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2019)McMurry OCB 3Ed Chapter 02 Polar covalent Bonds acids and Bases.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2019)McMurry OCB 3Ed Chapter 02-Sp Brief Introduction and Nomenclature of OCs.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2019)McMurry OCB 3Ed Chapter 07 & 08 alkenes and alkynes.pdf
- 运城学院:《环境化学》课程教学资源(教案讲义)理论教学大纲 Environmental Chemistry.pdf
- 运城学院:《环境化学》课程教学资源(教案讲义)实验二 土壤对铜离子的吸附.pdf
- 运城学院:《环境化学》课程教学资源(教案讲义)第一章 绪论 Environmental Chemistry.pdf
- 运城学院:《环境化学》课程教学资源(教案讲义)第三章 水环境化学.pdf
- 运城学院:《环境化学》课程教学资源(教案讲义)第四章 土壤环境化学 第一节 土壤的组成与性质.pdf
