西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2019)McMurry OCB 3Ed Chapter 07 & 08 alkenes and alkynes

有机北学 Organic Chemistry with BiologicalApplications.Edition John McMurry Chapter 07 eT 08 alkenes and alkynes Carbon-carbon double bonds are present in most organic and biological molecules,so a good understanding of the electrophilic addition reaction is needed. Key Notes Electrophile;Electrophilic addition; Carbocation stability;Conjugated dienes
Chapter 07 & 08 alkenes and alkynes Organic Chemistry with Biological Applications, 3rd Edition John McMurry Carbon–carbon double bonds are present in most organic and biological molecules, so a good understanding of the electrophilic addition reaction is needed. Key Notes Electrophile; Electrophilic addition; Carbocation stability; Conjugated dienes

有机化学网 Organic Chemistry with Biological Applications,3 Edition John McMurry Chapter 07 08 alkenes and alkynes Homework:P203:7.18,:7.21:P211e:7.47;7.50:7.51 P231:8.12a,P264c:8.34:8.36;8.47acd:8.60abcd By Junru Wang College of Chemistry and Pharmacy Room C206,Science Building .m i071
By Junru Wang College of Chemistry and Pharmacy Room C206, Science Building Tel: 87092829(O);Email: wangjr07@163 com Chapter 07 & 08 alkenes and alkynes Organic Chemistry with Biological Applications, 3rd Edition John McMurry Homework: P203:7.18; 7.21; P211e: 7.47; 7.50; 7.51; P231:8.12a; P264c:8.34 ;8.36; 8.47acd; 8.60abcd;

萜烯--天然存在的烯烃Terpenoids 公元1000年,波斯 ■水汽蒸馏-一植物一挥发油(精油) ·药物,香料,香水等 ■19世纪:-促进有机化学作为一门科学出现; ■i 萜烯类化合物 ·超过35000种天然萜烯类; ·骨架多样性--单萜,倍半萜,二萜,三萜,多萜 ■部位各异:玫瑰的花、薄荷的叶、檀香树干、桂树 皮、当归根、茴香果实、白豆蔻种子 ■功能活性有20多种:
萜烯---天然存在的烯烃Terpenoids 公元1000年,波斯 水汽蒸馏---植物—挥发油(精油) 药物,香料,香水等 19世纪:--促进有机化学作为一门科学出现; 萜烯类化合物 超过35000种天然萜烯类; 骨架多样性---单萜,倍半萜,二萜,三萜,多萜 部位各异:玫瑰的花、薄荷的叶、檀香树干、桂树 皮、当归根、茴香果实、白豆蔻种子 功能活性有20多种:

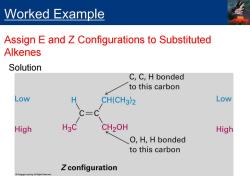
Worked Example Assign E and Z Configurations to Substituted Alkenes Solution C,C,H bonded to this carbon Low H CH(CH3)2 Low C=C High H3C CH2OH High O,H,H bonded to this carbon Z configuration
Solution Worked Example Assign E and Z Configurations to Substituted Alkenes

Main Contents Stability of alkenes Preparing alkenes:a preview EA Reactions of alkenes Carbocation structure and stability Reduction oxidation of alkenes Biological additions of Radicals to alkenes ■Conjugated Dienes ■Reactions of alkynes
Main Contents Stability of alkenes Preparing alkenes: a preview EA Reactions of alkenes Carbocation structure and stability Reduction & oxidation of alkenes Biological additions of Radicals to alkenes Conjugated Dienes Reactions of alkynes
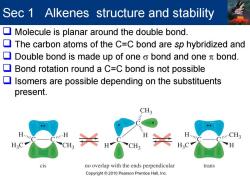
Sec 1 Alkenes structure and stability Molecule is planar around the double bond. 口 The carbon atoms of the C=C bond are sp hybridized and Double bond is made up of one o bond and one bond. Bond rotation round a C=C bond is not possible Isomers are possible depending on the substituents present. CH3 CH3 cis no overlap with the ends perpendicular trans Copyright 2010 Pearson Prentice Hall,Inc
Sec 1 Alkenes structure and stability Molecule is planar around the double bond. The carbon atoms of the C=C bond are sp hybridized and Double bond is made up of one σ bond and one π bond. Bond rotation round a C=C bond is not possible Isomers are possible depending on the substituents present
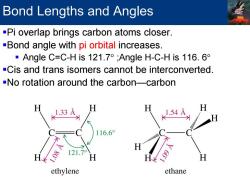
Bond Lengths and Angles -Pi overlap brings carbon atoms closer. -Bond angle with pi orbital increases. ·Angle C=C-His121.7°;Angle H-C-His116.6 -Cis and trans isomers cannot be interconverted -No rotation around the carbon-carbon 1.33 116.6° H ethylene ethane
Bond Lengths and Angles Pi overlap brings carbon atoms closer. Bond angle with pi orbital increases. Angle C=C-H is 121.7° ;Angle H-C-H is 116. 6° Cis and trans isomers cannot be interconverted. No rotation around the carbon—carbon
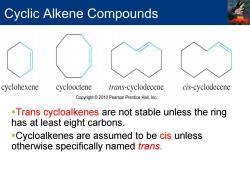
Cyclic Alkene Compounds cyclohexene cyclooctene trans-cyclodecene cis-cyclodecene Copyright 2010 Pearson Prentice Hall,Inc. -Trans cycloalkenes are not stable unless the ring has at least eight carbons. -Cycloalkenes are assumed to be cis unless otherwise specifically named frans
Cyclic Alkene Compounds Trans cycloalkenes are not stable unless the ring has at least eight carbons. Cycloalkenes are assumed to be cis unless otherwise specifically named trans

Stability of Alkenes Stability of cis and trans isomers Interconversion does not occur spontaneously but can be induced by strong acid H CH3 H3C、 CH3 Acid C=C catalyst H3C Trans (76%) Cis(24%) Steric strain cis-But-2-ene trans-But-2-ene
Stability of cis and trans isomers • Interconversion does not occur spontaneously but can be induced by strong acid Stability of Alkenes
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2019)McMurry OCB 3Ed Chapter 02-Sp Brief Introduction and Nomenclature of OCs.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2019)McMurry OCB 3Ed Chapter 02 Polar covalent Bonds acids and Bases.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2019)McMurry OCB 3Ed Chapter 01 Structure and Bonding(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2019)McMurry OCB 3Ed Chapter 00 Introduction to the course.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2018)McMurry OCB 3Ed Chapter 00 Introduction to the course(主讲:王俊儒).ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 12 Organohalides Nucleophilic Substitutions and Eliminations.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 09 aromatic compounds.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 06 an overview of organic Reactions.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 05 Stereochemistry at Tetrahedral Centers.pptx
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 04 Organic compounds cycloalkanes and their stereochemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 03 Organic compounds alkanes and their stereochemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 02-Sp Brief Introduction and Nomenclature of OCs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 00 Introduction to the course.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2017)McMurry OCB 3Ed Chapter 01 Structure and Bonding.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 21 & 22 Carboxylic Acids & Derivatives.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 17 & 18 Aromatic chemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 10 Alkynes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 08 & 09 Alkenes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2014)Chapter 06 Stereochemistry.ppt
- 运城学院:《环境化学》课程教学资源(教案讲义)理论教学大纲 Environmental Chemistry.pdf
- 运城学院:《环境化学》课程教学资源(教案讲义)实验二 土壤对铜离子的吸附.pdf
- 运城学院:《环境化学》课程教学资源(教案讲义)第一章 绪论 Environmental Chemistry.pdf
- 运城学院:《环境化学》课程教学资源(教案讲义)第三章 水环境化学.pdf
- 运城学院:《环境化学》课程教学资源(教案讲义)第四章 土壤环境化学 第一节 土壤的组成与性质.pdf
- 运城学院:《环境化学》课程教学资源(教案讲义)污染物质的生物转化.pdf
- 运城学院:《环境化学》课程教学资源(教案讲义)土壤中重金属的迁移和转化.pdf
- 运城学院:《环境化学》课程教学资源(教案讲义)环境化学授课教案 Environmental Chemistry.pdf
- 兰州交通大学:《有机化学》课程教学大纲 Organic Chemistry(打印版,负责人:柴兰琴).pdf
- 兰州交通大学:《有机化学》课程授课教案(打印版)第一章 有机化合物的结构和性质.pdf
- 兰州交通大学:《有机化学》课程授课教案(打印版)第二章 烷烃.pdf
- 兰州交通大学:《有机化学》课程授课教案(打印版)第三章 烯烃.pdf
- 兰州交通大学:《有机化学》课程授课教案(打印版)第四章 炔烃、二烯烃、红外光谱.pdf
- 兰州交通大学:《有机化学》课程授课教案(打印版)第五章 脂环烃.pdf
- 兰州交通大学:《有机化学》课程授课教案(打印版)第六章 单环芳烃.pdf
- 兰州交通大学:《有机化学》课程授课教案(打印版)第七章 多环芳烃和非苯芳烃.pdf
- 兰州交通大学:《有机化学》课程授课教案(打印版)第八章 立体化学.pdf
- 兰州交通大学:《有机化学》课程授课教案(打印版)第九章 卤代烃.pdf
- 兰州交通大学:《有机化学》课程授课教案(打印版)第十章 醇和醚.pdf
- 兰州交通大学:《有机化学》课程授课教案(打印版)第十一章 酚和醌.pdf
