西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 19 Ketones and Aldehydes
Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 19 Aldehydes and Ketones
Chapter 19 Aldehydes and Ketones Organic Chemistry, 6th Edition L. G. Wade, Jr

自成不精大好 Content 院 Carbonyl Structure Properties Preparation >Functional group transformations >Carbon-carbon bond formation >C-C Bond cleavage Reactions of Aldehydes and Ketones ●C=O ●o-H,-C
Content Carbonyl Structure & Properties Preparation Functional group transformations Carbon-carbon bond formation C-C Bond cleavage Reactions of Aldehydes and Ketones C=O -H, - C
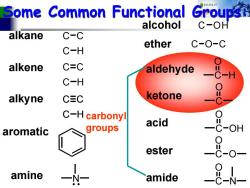
Some Common Functional Groups alcohol C-OH alkane C-C ether C-O-C C-H alkene C=C aldehyde C-H alkyne CEC ketone C-H carbonyl acid aromatic groups OH ester amine amide
Some Common Functional Groups: Some Common Functional Groups: C C C H alkane alkene C C C H alkyne C C C H aromatic alcohol C OH ether COC C O H aldehyde C O ketone C O OH acid C O ester O C O N amide N amine carbonyl groups
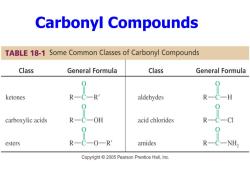
Carbonyl Compounds TABLE 18-1 Some Common Classes of Carbonyl Compounds Class General Formula Class General Formula ketones R R aldehydes R carboxylic acids OH acid chlorides esters -OR amides Copyright 2005 Pearson Prentice Hall,Inc
Carbonyl Compounds
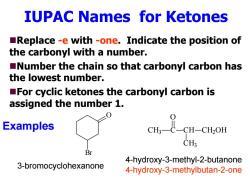
IUPAC Names for Ketones Replace -e with -one.Indicate the position of the carbonyl with a number. Number the chain so that carbonyl carbon has the lowest number. For cyclic ketones the carbonyl carbon is assigned the number 1. Examples CH: Br 4-hydroxy-3-methyl-2-butanone 3-bromocyclohexanone 4-hydroxy-3-methylbutan-2-one
IUPAC Names for Ketones Replace -e with -one. Indicate the position of the carbonyl with a number. Number the chain so that carbonyl carbon has the lowest number. For cyclic ketones the carbonyl carbon is assigned the number 1. O Br CH 3 C O CH CH 3 CH 2OH 3-bromocyclohexanone 4-hydroxy-3-methyl-2-butanone 4-hydroxy-3-methylbutan-2-one Examples

Naming Aldehydes IUPAC:Replace -e with -al The aldehyde carbon is number 1. If -CHO is attached to a ring,use the suffix -carbaldehyde. CHO Example 2-cyclopentenecarbaldehyde cyclopent-2-en-1-carbaldehyde
Naming Aldehydes IUPAC: Replace - e with -al. The aldehyde carbon is number 1. If -CHO is attached to a ring, use the suffix -carbaldehyde. Example CHO 2-cyclopentenecarbaldehyde cyclopent-2-en-1-carbaldehyde
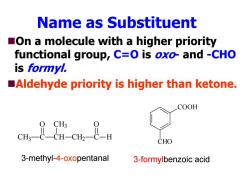
Name as Substituent On a molecule with a higher priority functional group,C=O is oxo-and -CHO is formyl. Aldehyde priority is higher than ketone. COOH CHO 3-methyl-4-oxopentanal 3-formylbenzoic acid
Name as Substituent On a molecule with a higher priority functional group, C=O is oxo- and -CHO is formyl. Aldehyde priority is higher than ketone. CH 3 C CH CH 3 CH 2 C H O O COOH CHO 3-methyl-4-oxopentanal 3-formylbenzoic acid
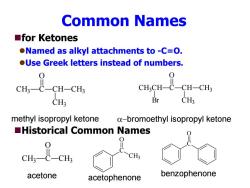
Common Names ■for Ketones Named as alkyl attachments to -C=O. OUse Greek letters instead of numbers. 0 0 CH3一C一CH-CH3 CH;CH-C-CH-CH3 CH3 Br CH: methyl isopropyl ketone a-bromoethyl isopropyl ketone Historical Common Names CH; acetone acetophenone benzophenone
Common Names for Ketones Named as alkyl attachments to -C=O. Use Greek letters instead of numbers. Historical Common Names CH 3 C O CH CH 3 CH 3 CH 3CH C O CH CH 3 CH 3 Br methyl isopropyl ketone bromoethyl isopropyl ketone CH 3 C O CH 3 C CH 3 O C O acetone acetophenone benzophenone

Aldehyde Common Names Use the common name of the acid. Drop -ic acid and add -aldehyde. 1 C:formic acid,formaldehyde 2 C's:acetic acid,acetaldehyde 3 C's:propionic acid,propionaldehyde 4 C's:butyric acid,butyraldehyde. Br B-bromobutyraldehyde 3-bromobutanal
Aldehyde Common Names Use the common name of the acid. Drop -ic acid and add -aldehyde. 1 C: formic acid, formaldehyde 2 C’s: acetic acid, acetaldehyde 3 C’s: propionic acid, propionaldehyde 4 C’s: butyric acid, butyraldehyde. CH 3 CH Br CH 2 C H O -bromobutyraldehyde 3-bromobutanal
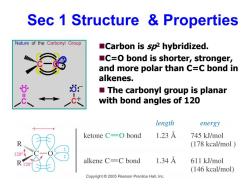
Sec 1 Structure Properties Nature of the Carbonyl Group Carbon is sp2 hybridized. C=O bond is shorter,stronger, and more polar than C=C bond in alkenes. The carbonyl group is planar with bond angles of 120 length energy ketone C=0 bond 1.23A 745 kJ/mol (178 kcal/mol alkene C=C bond 1.34A 611 kJ/mol (146 kcal/mol) Copyright 2005 Pearson Prentice Hall,Inc
Sec 1 Structure & Properties Carbon is sp2 hybridized. C=O bond is shorter, stronger, and more polar than C=C bond in alkenes. The carbonyl group is planar with bond angles of 120
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 08 & 09 Alkenes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 06 Stereochemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 05 The Study of Chemical Reactions.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 17 & 18 Aromatic chemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 04 Structure and Stereochemistry of Alkanes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 06 Stereochemistry(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 05 The Study of Chemical Reactions(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 04 Structure and Stereochemistry of Alkanes(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 03 Brief Introduction and Nomenclature of OC.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 02 Structure and Properties of OM.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 01 Introduction(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 11 Carboxylic acids and carboxylic acid derivatives.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 10 Aldehydes and ketones.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 09 Aromatic chemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 08 Alkenes and Alkynes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 07 Acids and Bases.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 06 reactions and mechanisms.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 05 Nucleophiles and Electrophiles.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 20 Amines.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 21 Carboxylic Acids.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 01 Introduction and Review(主讲:王俊儒).ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 14 & 15 Alcohols.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 17 & 18 Aromatic chemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 20 Amines.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 02 Structure and Properties of OMs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 03 Brief Introduction and Nomenclature of OCs.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 04 Structure and Stereochemistry of Alkanes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 05 The Study of Chemical Reactions.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 06 Stereochemistry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 08 & 09 Alkenes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 10 Alkynes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 19 Ketones and Aldehydes.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 21 Carboxylic Acids.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 22 Carboxylic Acid Derivatives.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 23 Condensation and Alpha Substitution of Carbonyl Compounds.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 24 Carbohydrates and Nucleic Acids.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 25 Amino Acids, Peptides and Proteins.ppt
