西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 09 Aromatic chemistry
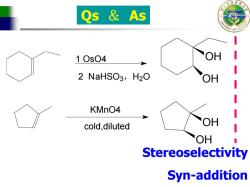
Qs As 10s04 OH 2 NaHSO3,H2O OH KMnO4 cold,diluted OH OH Stereoselectivity Syn-addition
College Qs & As of Science KMnO4 1 OsO4 2 NaHSO3,H2O cold,diluted OH OH OH OH Stereoselectivity Syn-addition
Section I -Aromatic chemistry
College of Science 西北农林科技大学理学院 Email: wangjr07@163.com Section I Aromatic chemistry

Section I 林 College Aromatic chemistry Aromaticity and reactivity ●Huckel rule Preparation and properties of Ar compounds Electrophilic substitutions Effects of substituents on electrophilic substitutions of substituted benzenes(E.S.) Synthesis of mono-,di-and tri-substituted benzenes Other reactions: .Oxidation and reduction
College of Science Section I Aromatic chemistry Aromaticity and reactivity zHückel rule Preparation and properties of Ar compounds Electrophilic substitutions Effects of substituents on electrophilic substitutions of substituted benzenes(E.S.) Synthesis of mono-, di- and tri-substituted benzenes Other reactions: zOxidation and reduction
I1 Aromaticity and Reactivity AdGif UNREGIST
College of Science I1 Aromaticity and Reactivity
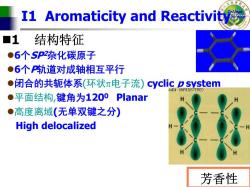
I1 Aromaticity and Reactivity ■1 结构特征 ●6个SP2杂化碳原子 ●6个P轨道对成轴相互平行 ●闭合的共轭体系(环状π电子流)cyclic p system AdGif-UNREGISTERED ●平面结构,键角为1200 Planar ●高度离域(无单双键之分) High delocalized 芳香性
College of Science I1 Aromaticity and Reactivity 1 结构特征 z 6 个SP2杂化碳原子 z 6 个 P轨道对成轴相互平行 z闭合的共轭体系 (环状 π电子流) cyclic p system z平面结构 ,键角为1200 Planar z高度离域 (无单双键之分 ) High delocalized 芳香性
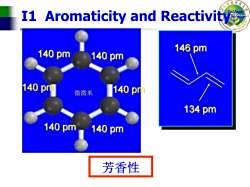
I1 Aromaticity and Reactivity 140pm 146pm 140pm 140pm 微微米 140pm 134pm 140pm140pm 芳香性
College of Science I1 Aromaticity and Reactivity 140 pm 140 pm 140 pm 140 pm 140 pm 140 pm 146 pm 134 pm 微微米 芳香性
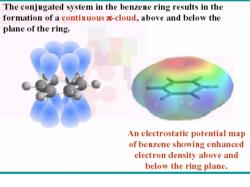
The conjugated system in the benzene ring results in the formation of a continuous -cloud,above and below the plane of the ring. An electrostatic potential map of benzene showing enhanced electron density above and below the ring plane
College of Science
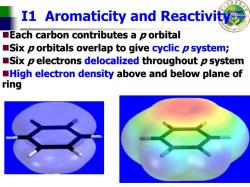
I1 Aromaticity and Reactivity Each carbon contributes a p orbital Six p orbitals overlap to give cyclic p system; Six p electrons delocalized throughout p system High electron density above and below plane of ring
College of Science I1 Aromaticity and Reactivity Each carbon contributes a Each carbon contributes a p orbital orbital Six p orbitals orbitals overlap to give overlap to give cyclic p system ; Six p electrons electrons delocalized delocalized throughout throughout p system High electron density High electron density above and below plane of above and below plane of ring

I1 Aromaticity and Reactivity ■2.反应性 ●不易加成(苯环骨架不断开) ●发生取代(亲电取代E.S) ●侧链反应 ■3.芳香性化合物结构特征 >大的不饱和度(2≥4)2=? >(环状)平面分子 >易进行亲电取代反应 芳香性
College of Science I1 Aromaticity and Reactivity 2. 反应性 z不易加成 (苯环骨架不断开 ) z发生取代 (亲电取代 E.S) z侧链反应 3. 芳香性化合物结构特征 ¾大的不饱和度 ( Ω ≧4) Ω=? ¾ (环状 )平面分子 ¾易进行亲电取代反应 芳香性
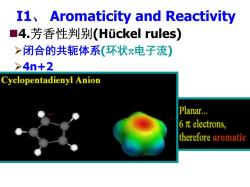
I1,Aromaticity and Reactivity ■4.芳香性判别(Huckel rules) >闭合的共轭体系(环状π电子流) >4n+2 Cyclopentadienyl Anion Planar... 6πelectrons, therefore aromatic
I1、 Aromaticity and Reactivity 4.芳香性判别(Hückel rules) ¾闭合的共轭体系(环状π电子流) ¾4n+2
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 08 Alkenes and Alkynes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 07 Acids and Bases.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 06 reactions and mechanisms.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 05 Nucleophiles and Electrophiles.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 04 stereochemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 03 functional groups.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 02 Alkanes and cycloalkane.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 01 Structure And Bonding(主讲:王俊儒).pdf
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Proton NMR Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Nomenclature of Saturated Hydrocarbons.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared(IR)spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Functional Groups.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ethers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Enols and Enolates.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 10 Aldehydes and ketones.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 11 Carboxylic acids and carboxylic acid derivatives.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 01 Introduction(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 02 Structure and Properties of OM.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 03 Brief Introduction and Nomenclature of OC.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 04 Structure and Stereochemistry of Alkanes(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 05 The Study of Chemical Reactions(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 06 Stereochemistry(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 04 Structure and Stereochemistry of Alkanes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 17 & 18 Aromatic chemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 05 The Study of Chemical Reactions.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 06 Stereochemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 08 & 09 Alkenes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 19 Ketones and Aldehydes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 20 Amines.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 21 Carboxylic Acids.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 01 Introduction and Review(主讲:王俊儒).ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程PPT教学课件(2013)Chapter 14 & 15 Alcohols.ppt
