西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 02 Alkanes and cycloalkane
Section B Alkanes and cycloalkanes 烷烃与环烷烃
Section B Alkanes and cycloalkanes 烷烃与环烷烃

Key Notes in this chapter B1 Alkanes B2 Cycloalkanes B3 Three dimension structure Conformation
Key Notes in this chapter B1 Alkanes B2 Cycloalkanes B3 Three dimension structure Conformation

重点 EST ABF 必1.烷烃与环烷烃命名 2.烷烃的结构特征 3.立体结构表示方法 4.环烷烃结构特征
重点 1.烷烃与环烷烃命名 2.烷烃的结构特征 3.立体结构表示方法 4.环烷烃结构特征
B1 Definition Alkanes are organic molecules consisting solely of carbon and hydrogen atoms linked by single o bonds. >All the carbon atoms are tetrahedral and sp3 hybridized. >Alkanes are stable molecules and unreactive to most chemical reagents. >Saturated hydrocarbons,straight chain or acyclic carbons Cycloalkanes are cyclic alkane structures. >They have the general formula CnH2n. >Most cycloalkanes are unreactive to chemical reagents
B1 Definition Alkanes are organic molecules consisting solely of carbon and hydrogen atoms linked by single σ bonds. ¾All the carbon atoms are tetrahedral and sp3 hybridized. ¾Alkanes are stable molecules and unreactive to most chemical reagents. ¾Saturated hydrocarbons,straight chain or acyclic carbons Cycloalkanes are cyclic alkane structures. ¾They have the general formula C n H2n. ¾Most cycloalkanes are unreactive to chemical reagents
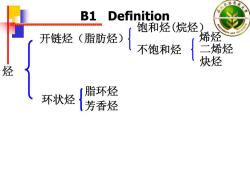
衣林 B1 Definition 饱和烃(烷烃 开链烃(脂肪烃) 烯烃 不饱和烃 二烯烃 炔烃 烃 脂环烃 环状烃 芳香烃
烃 开链烃(脂肪烃) 饱和烃(烷烃) 不饱和烃 烯烃 二烯烃 炔烃 环状烃 脂环烃 芳香烃 B1 Definition
Alkanes Cycloalkanes Alkanes are sometimes referred to as straight chain or acyclic alkanes to dis-tinguish them from cycloalkanes or alicyclic(脂环族的)compounds. The most commonly encountered cycloalkane in organic chemistry is the six-membered ring (cyclohexane
Alkanes & Cycloalkanes Alkanes are sometimes referred to as straight chain or acyclic alkanes to dis-tinguish them from cycloalkanes or alicyclic(脂环族的) compounds. The most commonly encountered cycloalkane in organic chemistry is the six-membered ring (cyclohexane)

Alkanes Cycloalkanes 十十 small three-and four-membered rings are reactive and behave like alkenes. Such cyclic structures are highly strained since it is impossible for the carbon atoms to adopt their preferred tetrahedral shape
Alkanes & Cycloalkanes small three- and four-membered rings are reactive and behave like alkenes. Such cyclic structures are highly strained since it is impossible for the carbon atoms to adopt their preferred tetrahedral shape
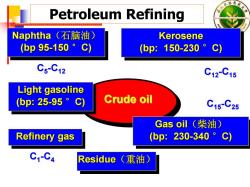
东林 Petroleum Refining Naphtha(石脑油) Kerosene (bp95-150°c) (bp:150-230。C) C5-C12 C12C15 Light gasoline (bp:25-95。C) Crude oil C15-C25 Gas oil(柴油) Refinery gas (bp:230-340。C) C1-C4 Residue (重油)
Petroleum Refining Naphtha(石脑油) (bp 95-150 °C) Naphtha Naphtha (石脑油 ) (bp 95 -150 °C) Kerosene (bp: 150-230 °C) Kerosene Kerosene (bp: 150 -230 °C) Crude oil Crude oil Refinery gas Refinery gas Refinery gas Light gasoline (bp: 25-95 °C) Light gasoline Light gasoline (bp: 25 -95 °C) C 5 - C12 C12 - C15 Gas oil(柴油) (bp: 230-340 °C) Gas oil Gas oil (柴油) (bp: 230 -340 °C) C15 - C25 C 1 - C 4 Residue Residue Residue ((重油) 重油)
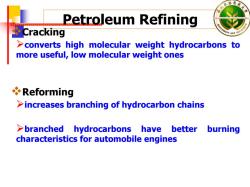
林 Petroleum Refining Cracking >converts high molecular weight hydrocarbons to more useful,low molecular weight ones Reforming >increases branching of hydrocarbon chains >branched hydrocarbons have better burning characteristics for automobile engines
Petroleum Refining Cracking ¾converts high molecular weight hydrocarbons to more useful, low molecular weight ones Reforming ¾increases branching of hydrocarbon chains ¾branched hydrocarbons have better burning characteristics for automobile engines

B2 Stereostructure Drawing 立体结构 与立体结构表示方法
B2 Stereostructure Drawing 立体结构 与立体结构表示方法
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 01 Structure And Bonding(主讲:王俊儒).pdf
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared Spectroscopy and Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Proton NMR Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Nomenclature of Saturated Hydrocarbons.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared(IR)spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Functional Groups.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ethers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Enols and Enolates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conjugated Dienes and U.V. Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conformational Analysis.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Chemistry of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acids Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Benzene and Aromaticity(2011).ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 03 functional groups.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 04 stereochemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 05 Nucleophiles and Electrophiles.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 06 reactions and mechanisms.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 07 Acids and Bases.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 08 Alkenes and Alkynes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 09 Aromatic chemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 10 Aldehydes and ketones.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 11 Carboxylic acids and carboxylic acid derivatives.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 01 Introduction(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 02 Structure and Properties of OM.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 03 Brief Introduction and Nomenclature of OC.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 04 Structure and Stereochemistry of Alkanes(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 05 The Study of Chemical Reactions(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 06 Stereochemistry(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 07 Alkyl Halides Nucleophilic Substitution and Elimination(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 04 Structure and Stereochemistry of Alkanes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 17 & 18 Aromatic chemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 05 The Study of Chemical Reactions.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2012)Chapter 06 Stereochemistry.pdf
