《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Proton NMR Spectroscopy
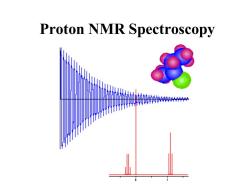
Proton NMR Spectroscopy
Proton NMR Spectroscopy
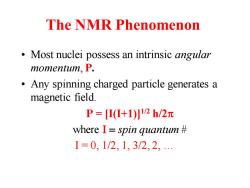
The NMR Phenomenon Most nuclei possess an intrinsic angular momentum,P. Any spinning charged particle generates a magnetic field. P=Π(+1)2h/2元 where I spin quantum I=0,1/2,1,3/2,2
The NMR Phenomenon • Most nuclei possess an intrinsic angular momentum, P. • Any spinning charged particle generates a magnetic field. P = [I(I+1)]1/2 h/2p where I = spin quantum # I = 0, 1/2, 1, 3/2, 2, …

Which nuclei have a "spin"? If mass and atomic are both even,I =0 and the nucleus has no spin. e.g.Carbon-12,Oxygen-16 For each nucleus with a spin,the of allowed spin states can be quantized: For a nucleus with I,there are 21+1 allowed spin states. H,13C,19F,31P all have I 1/2 △E=yh/2π)Bo
Which nuclei have a “spin”? • If mass # and atomic # are both even, I = 0 and the nucleus has no spin. e.g. Carbon-12, Oxygen-16 • For each nucleus with a spin, the # of allowed spin states can be quantized: • For a nucleus with I, there are 2I + 1 allowed spin states. 1H, 13C, 19F, 31P all have I = 1/2 DE = g(h/2p)Bo

Spin states split in the presence of Bo -1/2 antiparallel E +1/2 parallel no field applied field Bo
Spin states split in the presence of B0 no field applied field E +1/2 parallel -1/2 antiparallel Bo
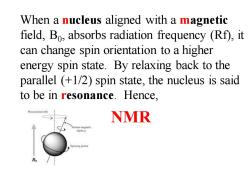
When a nucleus aligned with a magnetic field,Bo,absorbs radiation frequency (Rf),it can change spin orientation to a higher energy spin state.By relaxing back to the parallel (+1/2)spin state,the nucleus is said to be in resonance.Hence, NMR B
When a nucleus aligned with a magnetic field, B0 , absorbs radiation frequency (Rf), it can change spin orientation to a higher energy spin state. By relaxing back to the parallel (+1/2) spin state, the nucleus is said to be in resonance. Hence, NMR

Presence of Magnetic Field No field With field
Presence of Magnetic Field
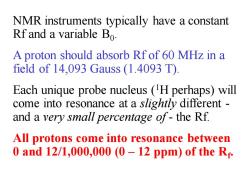
NMR instruments typically have a constant Rf and a variable Bo. A proton should absorb Rf of 60 MHz in a field of 14,093 Gauss (1.4093 T) Each unique probe nucleus (H perhaps)will come into resonance at a slightly different and a very small percentage of-the Rf. All protons come into resonance between 0 and 12/1,000,000(0-12 ppm)of the R
NMR instruments typically have a constant Rf and a variable B0 . A proton should absorb Rf of 60 MHz in a field of 14,093 Gauss (1.4093 T). Each unique probe nucleus (1H perhaps) will come into resonance at a slightly different - and a very small percentage of - the Rf. All protons come into resonance between 0 and 12/1,000,000 (0 – 12 ppm) of the Rf

Energy Difference (AE)Between Two Different Spin States of a Nucleus With I=1/2 -1/2 antiparallel E 100 MHZ 200 MHz 300 MHz 400 MHz parallel +1/2 23,500 47,000 70,500 104,000 inc.magnetic field strength,Gauss Bo
Energy Difference (DE) Between Two Different Spin States of a Nucleus With I=1/2 +1/2 -1/2 E 100 MHz 200 MHz 300 MHz 400 MHz 23,500 47,000 70,500 104,000 parallel antiparallel inc. magnetic field strength, Gauss B0

What Does an NMR Spectrum Tell You? .of chemically unique H's in the molecule of signals The types of H's that are present e.g. aromatic,vinyl,aldehyde .. chemical shift The number of each chemically unique H integration The H's proximity to eachother spin-spin splitting
What Does an NMR Spectrum Tell You? • # of chemically unique H’s in the molecule # of signals • The types of H’s that are present e.g. aromatic, vinyl, aldehyde … chemical shift • The number of each chemically unique H integration • The H’s proximity to eachother spin-spin splitting
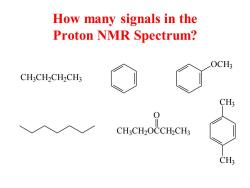
How many signals in the Proton NMR Spectrum? OCH3 CH3CH2CH2CH3 CH3 0 CH.CH2OCCHCH3 CH3
How many signals in the Proton NMR Spectrum? C H3 C H2 C H2 C H3 OCH3 C H3 C H2 OCCH2 C H3 O CH3 CH3
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Nomenclature of Saturated Hydrocarbons.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared(IR)spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Functional Groups.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ethers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Enols and Enolates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conjugated Dienes and U.V. Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conformational Analysis.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Chemistry of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acids Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Benzene and Aromaticity(2011).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkynes McMurry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkyl Halides from Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Overview.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alcohols-structure and synthesis 2.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared Spectroscopy and Mass Spectrometry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 01 Structure And Bonding(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 02 Alkanes and cycloalkane.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 03 functional groups.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 04 stereochemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 05 Nucleophiles and Electrophiles.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 06 reactions and mechanisms.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 07 Acids and Bases.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 08 Alkenes and Alkynes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 09 Aromatic chemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 10 Aldehydes and ketones.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 11 Carboxylic acids and carboxylic acid derivatives.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 01 Introduction(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 02 Structure and Properties of OM.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 03 Brief Introduction and Nomenclature of OC.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 04 Structure and Stereochemistry of Alkanes(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 05 The Study of Chemical Reactions(打印版).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 06 Stereochemistry(打印版).pdf
