《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Nomenclature of Saturated Hydrocarbons

Nomenclature of Saturated Hydrocarbons
Nomenclature of Saturated Hydrocarbons

Some Simple Alkanes(C H2n+2) HH HHH CH3 H一C-HH-C-C-H H-C-C-C-H CH3一CH一CH H HH HHH HHH H methane,CHa ethane,C2H propane,CHg butane,CHo isobutane,CHo CH3 CH3 CH3-CH2一CH2-CH2-CH3 CH3-CH-CH2-CH3 CH3-C-CH3 or H-(CH2方H CH3 pentane,CsH2 isopentane,CsH2 neopentane,CsH12
Some Simple Alkanes (CnH2n+2)
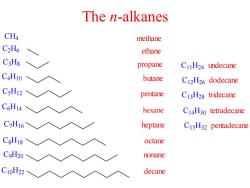
The n-alkanes CH4 methane C2H6 ethane C3Hg propane C1H24 undecane C4H10 butane C12H26 dodecane C5H12 pentane C13H28 tridecane C6H14 hexane C14H30 tetradecane C7H16 heptane CIsH32 pentadecane C8H18 octane CoH20 nonane C10H22 decane
The n-alkanes C H4 C2H6 C3H8 C4H10 C5H12 C6H14 C7H16 C8H18 C9H20 C10H22 methane ethane propane butane pentane hexane heptane octane nonane decane C11H24 undecane C12H26 dodecane C13H28 tridecane C14H30 tetradecane C15H32 pentadecane
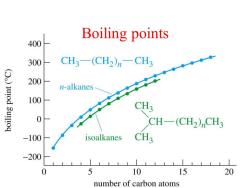
Boiling points 400 300 CH3-(CH2)n一CHg ()uod 3u!!oq 200 n-alkanes 100 CH3 0 CH-(CH2)CH3 -100 isoalkanes CH3 -200 0 5 10 15 20 number of carbon atoms
Boiling points
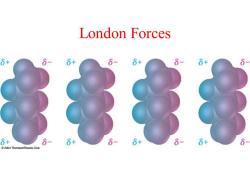
London Forces 8+ 8-6+ 8-8+ 6-6+ 8 8+ 6+ 6- 6+ 6+ @2004 Thomson/Brooks Cole
London Forces
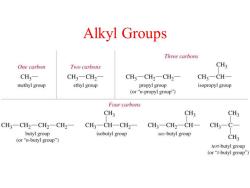
Alkyl Groups Three carbons One carbon Two carbons CH3 CH3- CH3-CH2- CH3一CH2一CH2一 CH3-CH- methyl group ethyl group propyl group isopropyl group (or“n-propyl group") Four carbons CH3 CH3 CH3 CH3-CH2-CH2-CH2一 CH3-CH-CH2- CH3-CH2-CH- CH3-C- butyl group isobutyl group sec-butyl group (or“-butyl group") CH3 tert-butyl group (or“t-butyl group")
Alkyl Groups
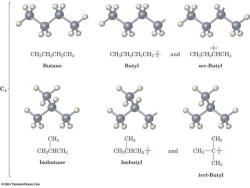
CH.CH2CH2CH3 CHCH2CH2CH2之 and CHCH2CHCH3 Butane Butyl sec-Butyl CH CH3 CH3 CH.CHCH3 CHgCHCH2 and CH3-C Isobutane Isobutyl CHs tert-Butyl 2004 Thomson/Brooks Cole
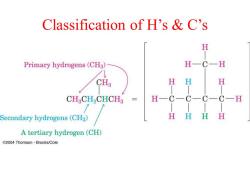
Classification of H's C's H Primary hydrogens(CH3) H一C一 CH3 H H H CH2CH2CHCH3 H一C一CC一CH Secondary hydrogens(CH2) H HH H A tertiary hydrogen(CH) C2004 Thomson-Brooks/Cole
Classification of H’s & C’s

The I.U.P.A.C.Rules Find longest carbon chain Number chain from end closest to nearest branch (with 2 different longest chains,use one with most substituents) Give alkyl groups attached to the longest chain a name and a number Multiple alkyl groups named alphabetically Multiple groups that are the same:di(2),tri(3), tetra(4),penta(5),hexa(6) ·Halogens are name“halo”groups-fluoro,.chloro, bromo,iodo
The I.U.P.A.C. Rules • Find longest carbon chain • Number chain from end closest to nearest branch (with 2 different longest chains, use one with most substituents) • Give alkyl groups attached to the longest chain a name and a number • Multiple alkyl groups named alphabetically • Multiple groups that are the same: di(2), tri(3), tetra(4), penta(5), hexa(6) • Halogens are name “halo” groups – fluoro, chloro, bromo, iodo
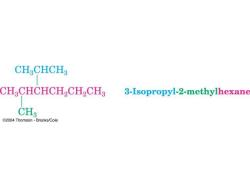
CH CHCH3 CHaCHCHCH2 CH2CH3 3-Isopropyl-2-methylhexane CH3 @2004 Thomson-Brooks/Cole
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared(IR)spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Functional Groups.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ethers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Enols and Enolates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conjugated Dienes and U.V. Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conformational Analysis.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Chemistry of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acids Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Benzene and Aromaticity(2011).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkynes McMurry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkyl Halides from Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Overview.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alcohols-structure and synthesis 2.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Structure and Bonding of Organic Molecules.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)CHM 201 Introduction and Review - Structure and Bonding.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Proton NMR Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared Spectroscopy and Mass Spectrometry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 01 Structure And Bonding(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 02 Alkanes and cycloalkane.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 03 functional groups.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 04 stereochemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 05 Nucleophiles and Electrophiles.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 06 reactions and mechanisms.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 07 Acids and Bases.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 08 Alkenes and Alkynes.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 09 Aromatic chemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 10 Aldehydes and ketones.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 11 Carboxylic acids and carboxylic acid derivatives.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 01 Introduction(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 02 Structure and Properties of OM.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 03 Brief Introduction and Nomenclature of OC.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2010)Chapter 04 Structure and Stereochemistry of Alkanes(打印版).pdf
