《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Structure and Bonding of Organic Molecules

Structure and Bonding of Organic Molecules Orbital Theory Hybridization and Geometry Polarity Functional Groups
Structure and Bonding of Organic Molecules Orbital Theory Hybridization and Geometry Polarity Functional Groups

CH3 CH3CH20 CH3O CH3 CH2CH2CH3 CH3 OH 0 CH3 Rofecoxib Sildenafil Oxycodone (Vioxx) (Viagra) (OxyContin) CH3 H S CH3 CH3 HO H H C02 Cholesterol Benzylpenicillin 2007 Thomson Higher Education
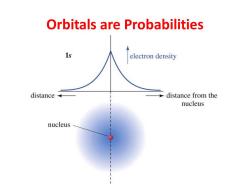
Orbitals are Probabilities 1s electron density distance distance from the nucleus nucleus
Orbitals are Probabilities
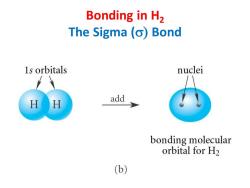
Bonding in H2 The Sigma (o)Bond 1s orbitals nuclei add bonding molecular orbital for H2 (b)
Bonding in H2 The Sigma (s) Bond
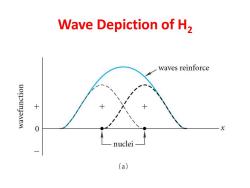
Wave Depiction of H2 waves reinforce uonounJaneM 0 X nuclei (a)
Wave Depiction of H2
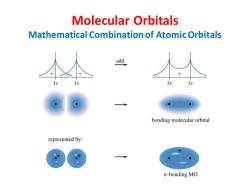
Molecular Orbitals Mathematical Combination of Atomic Orbitals add bonding molecular orbital represented by: o-bonding MO
Molecular Orbitals Mathematical Combination of Atomic Orbitals
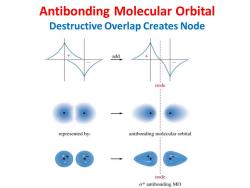
Antibonding Molecular Orbital Destructive Overlap Creates Node add node represented by: antibonding molecular orbital node σantibonding MO
Antibonding Molecular Orbital Destructive Overlap Creates Node
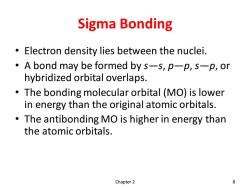
Sigma Bonding Electron density lies between the nuclei. A bond may be formed by s-s,p-p,s-p,or hybridized orbital overlaps. The bonding molecular orbital (MO)is lower in energy than the original atomic orbitals. The antibonding MO is higher in energy than the atomic orbitals. Chapter 2 8
Sigma Bonding • Electron density lies between the nuclei. • A bond may be formed by s—s, p—p, s—p, or hybridized orbital overlaps. • The bonding molecular orbital (MO) is lower in energy than the original atomic orbitals. • The antibonding MO is higher in energy than the atomic orbitals. Chapter 2 8
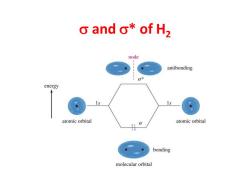
o and o*of H2 node antibonding energy atomic orbital 0 atomic orbital bonding molecular orbital
s and s* of H2
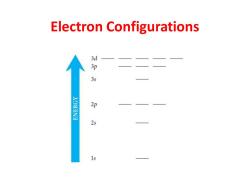
Electron Configurations d 3s 2p 2s 1s
Electron Configurations
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)CHM 201 Introduction and Review - Structure and Bonding.pptx
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 21 Carboxylic acid Derivatives.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 20 Carboxylic Acids.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 19 Amines.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 18 Ketones and Aldehydes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 17 Reactions of Aromatic Compounds.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 16 Aromatic Compounds.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 15 Conjugated Systems.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 14 Ethers and Epoxides.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 13 Nuclear Magnetic Resonance(NMR)Spectroscopy.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 11 Reactions of Alcohols.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 10 Synthesis and Structure of Alcohols.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 09 Alkynes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 08 Reactions of Alkenes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 07 Structure and Synthesis of Alkenes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 06 Alkyl Halides.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 05 Stereochemistry.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 04 Rates & Kinetics.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 03 Alkanes.pdf
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alcohols-structure and synthesis 2.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Overview.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkyl Halides from Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkynes McMurry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Benzene and Aromaticity(2011).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acids Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Chemistry of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conformational Analysis.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conjugated Dienes and U.V. Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Enols and Enolates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ethers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Functional Groups.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared(IR)spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Nomenclature of Saturated Hydrocarbons.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Organic Reactions.ppt
