《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 03 Alkanes

Alkanes Alkanes are the simplest organic molecules,they only contain C and hydrogen,and only contain single bonds. Compounds that have the maximum number of bonded hydrogens,are said to be saturated. Alkanes are saturated hydrocarbons,with a general Formula:CH22 The simplest members of this group are the n-alkanes. H H-C-H methane C H4 H HH H-C-C-H ethane C2He H HH H-C-C-C-H propane C3Ha H HH HHHH H-C-C-C-C-H butane C4H10 HHHH HHHHH H-C-C-C-C-C-H pentane CsH12 HHHHH Ch03 Alkanes (landscape) Page I
Ch03 Alkanes (landscape) Page 1 Alkanes Alkanes are the simplest organic molecules, they only contain C and hydrogen, and only contain single bonds. Compounds that have the maximum number of bonded hydrogens, are said to be saturated. Alkanes are saturated hydrocarbons, with a general Formula: CnH2n+2 The simplest members of this group are the n-alkanes. H C H H H H C H H C H H H H C H C H H H C H H H H C H C H H H C H H C C C H C H H H C H H C H H H H H H H H H methane C1H4 ethane C2H6 propane C3H8 butane C4H10 pentane C5H12

The n-alkanes are straight chain molecules,but there are also branched alkanes(isomers). CH CH,一CH,-CH,-CH3 CH,一CH-CH butane (n-butane) isobutane CH, CH CH,一CH2CH2一CH2-CH3 CH;CH-CH,-CH, CH;C-CH, pentane (n-pentane) isopentane CH neopentane Any series that differs only by an increasing number of-CH2-groups is known as a Homologous series. The individual members are said to be homologs of each other. Ch03 Alkanes (landscape) Page 2
Ch03 Alkanes (landscape) Page 2 The n-alkanes are straight chain molecules, but there are also branched alkanes (isomers). Any series that differs only by an increasing number of –CH2- groups is known as a Homologous series. The individual members are said to be homologs of each other

Nomenclature of Alkanes There are two general types of nomenclature trivial names (acetone,acetic acid) IUPAC System(propanone,ethanoic acid) IUPAC or Systematic Names The systematic way to(UNAMBIGUOUSLY)name all organic compounds. For alkanes: (1)Find the longest continuous chain of carbon atoms.This is the base name of the compound. (2)Number the longest chain beginning with the end nearest a substituent. (3)Name the substituent groups attached to the longest chain as alkyl groups.Also state the location of each alkyl group according to its numbered carbon on the main chain. (4)When two or more substituents are present,list them in alphabetical order.If two or more of the same alkyl groups are present,use the prefixes di-,tri-etc to avoid repetition. Examples: 1 CH2CH3 CH,CH H3C-CH-CH2-CH2-CH3 CH CH-CH2一CH2一CH3 3 4 6 3-methylhexane Ch03 Alkanes (landscape) Page 3
Ch03 Alkanes (landscape) Page 3 Nomenclature of Alkanes There are two general types of nomenclature: trivial names (acetone, acetic acid) IUPAC System (propanone, ethanoic acid) IUPAC or Systematic Names The systematic way to (UNAMBIGUOUSLY) name all organic compounds. For alkanes: (1) Find the longest continuous chain of carbon atoms. This is the base name of the compound. (2) Number the longest chain beginning with the end nearest a substituent. (3) Name the substituent groups attached to the longest chain as alkyl groups. Also state the location of each alkyl group according to its numbered carbon on the main chain. (4) When two or more substituents are present, list them in alphabetical order. If two or more of the same alkyl groups are present, use the prefixes di-, tri- etc to avoid repetition. Examples:

If there are two chains of equal length,choose the chain that has the highest number of substituents. CH CH: CH3 CH一CH CH: CH- CH2 CH- CH-CH,CH CH- CH- CHCH; CH3 CH-CH3 CH,CH-CH CH CH3 wrong correct seven-carbon chain,but only three substituents seven-carbon chain,four substituents Numbering starts at the end nearest a substituent so that the alkyl substituents have as low numbers as possible CH methyl CH CH CH2 CH. CH ethyl CH CH,CH: CH- CH CHCH: CH CH- CH CH, CH- CH CH. methyl CH, methyl incorrect correct 3-ethyl-2,4,5-trimethylheptane Ch03 Alkanes (landscape) Page 4
Ch03 Alkanes (landscape) Page 4 If there are two chains of equal length, choose the chain that has the highest number of substituents. Numbering starts at the end nearest a substituent so that the alkyl substituents have as low numbers as possible

Alkyl groups are named by replacing the-ane suffix of the alkane name with-yl E.g CH3CH3 Ethane CH3CH2- Ethyl group CH;CH2CH3Propane CH:CH2CH2- Propyl group Common branched alkyl groups have trivial names: CH3 CH3 CH3 CHgCH2-CH2CH2>HgC-CH-CH2-CHgCH2-CH HgC-C butyl isobutyl sec-butyl CH3 tert-butyl t-butyl The names sec and tert are short for secondary and tertiary,referring to the degree of alkyl substitution. H R-C-R-C- R-C H H R Primary Secondary Tertiary 1°carbon2°carbon3°carbon Prefixes are used when there are more than one type of alkyl substituent Di=2 Tri=3 Tetra=4 Penta =5 The prefixes do not count when alphabetizing. Ch03 Alkanes (landscape) Page 5
Ch03 Alkanes (landscape) Page 5 Alkyl groups are named by replacing the –ane suffix of the alkane name with –yl. E.g. CH3CH3 Ethane CH3CH2- Ethyl group CH3CH2CH3 Propane CH3CH2CH2- Propyl group Common branched alkyl groups have trivial names: The names sec and tert are short for secondary and tertiary, referring to the degree of alkyl substitution. Prefixes are used when there are more than one type of alkyl substituent Di = 2 Tri = 3 Tetra = 4 Penta = 5 The prefixes do not count when alphabetizing. R C H H R C R H R C R R Primary 1 o carbon Secondary 2 o carbon Tertiary 3 o carbon

The below compound is 3-ethyl-2,4,5-trimethylheptane CH3 H3C -CH2 HC -CH2CHg CH3 CH3 CH,- CH-CH2 CH-CH- CH,CH3 CH CH- CH CH 3-ethyl-2,4,5-trimethylheptane Ch03 Alkanes (landscape) Page 6
Ch03 Alkanes (landscape) Page 6 The below compound is 3-ethyl-2,4,5-trimethylheptane CH3 CH2 H C HC H3C H C H3C HC CH2CH3 CH3 CH3

Complex Substituents These are named as follows: (a)The base alkyl group is numbered starting with the carbon bonded to the main chain. (b) The substituents are listed with the appropriate numbers,and parentheses are used to separate the substituent name. CH3CH3 -C-CH-CH-CH3 CH3 a(1,1,3-trimethylbutyl)group Properties of Alkanes Natural gas,gasoline,oils and paraffin wax are all alkanes,and so alkanes are often used as fuels,lubricants and solvents. Alkanes are non-polar,and are said to be Hydrophobic(water hating)since they do not dissolve in water. Typically the density of alkanes is around 0.7g/ml,and so when an alkane and water are mixed,they will form two separate phases,with the alkane on top.(Oil floats on water). Ch03 Alkanes (landscape) Page 7
Ch03 Alkanes (landscape) Page 7 Complex Substituents These are named as follows: (a) The base alkyl group is numbered starting with the carbon bonded to the main chain. (b) The substituents are listed with the appropriate numbers, and parentheses are used to separate the substituent name. Properties of Alkanes Natural gas, gasoline, oils and paraffin wax are all alkanes, and so alkanes are often used as fuels, lubricants and solvents. Alkanes are non-polar, and are said to be Hydrophobic (‘water hating’) since they do not dissolve in water. Typically the density of alkanes is around 0.7g/ml, and so when an alkane and water are mixed, they will form two separate phases, with the alkane on top. (Oil floats on water). CH3 C-CH2 CH3 CH-CH3 CH3 a (1,1,3-trimethylbutyl) group

Reactivity of Alkanes Shorter chain alkanes are obtained commercially by the 'catalytic cracking'of larger chain alkanes such as crude oil or petroleum refining H2 入A入入Heat"入/ C12H26 Catalyst CsH12 入N/ C7H16 The process of using hydrogen gas to ensure all the products are alkanes is called Hydrocracking. In general,alkanes are chemically unreactive,although reactions do occur under forcing conditions Combustion Alkanes are converted to carbon dioxide and water at high temperatures. CHgCH2CH3 +502-3CO2 4H2O (This is why alkanes are good fuels). Halogenation Alkanes will react with halogens(F2,Cl2,Br2,12)under conditions of heat or light. CH4 Cl2- CH3Cl CH2Cl2 CHCI3 CCl4 HCI Mixtures of alkyl halides are formed. Ch03 Alkanes (landscape) Page 8
Ch03 Alkanes (landscape) Page 8 Reactivity of Alkanes Shorter chain alkanes are obtained commercially by the ‘catalytic cracking’ of larger chain alkanes such as crude oil or petroleum refining. The process of using hydrogen gas to ensure all the products are alkanes is called Hydrocracking. In general, alkanes are chemically unreactive, although reactions do occur under forcing conditions. Combustion Alkanes are converted to carbon dioxide and water at high temperatures. (This is why alkanes are good fuels). Halogenation Alkanes will react with halogens (F2, Cl2, Br2, I2) under conditions of heat or light. Mixtures of alkyl halides are formed. H2 Heat C12H26 Catalyst C5H12 C7H16 CH4 + Cl2 CH3Cl + CH2Cl2 + CHCl3 + CCl4 + HCl CH3CH2CH3 + 5O2 3CO2 + 4H2O

Structure and Conformation of Alkanes Because alkanes are saturated,they exemplify sphybridized carbon,and most of the fundamental structural and conformational properties of organic molecules can be found in structures of alkanes. E.g.Methane,CH4,is the simplest alkane. H 109.5 1.09A H H It is perfectly tetrahedral,with bond angles of 109.5 The C-H bond length is 1.09A.(1.09 x 1010 m). Ch03 Alkanes (landscape) Page 9
Ch03 Alkanes (landscape) Page 9 Structure and Conformation of Alkanes Because alkanes are saturated, they exemplify sp3 hybridized carbon, and most of the fundamental structural and conformational properties of organic molecules can be found in structures of alkanes. E.g. Methane, CH4, is the simplest alkane. It is perfectly tetrahedral, with bond angles of 109.5o . The C-H bond length is 1.09Å. (1.09 x 10-10 m)
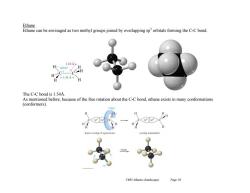
Ethane Ethane can be envisaged as two methyl groups joined by overlapping sporbitals forming the C-C bond. 1.10A H-109.6 H H 1.54A H The C-C bond is 1.54A As mentioned before,because of the free rotation about the C-C bond,ethane exists in many conformations (conformers). near overlap of sigma bon Ch03 Alkanes (landscape) Page 10
Ch03 Alkanes (landscape) Page 10 Ethane Ethane can be envisaged as two methyl groups joined by overlapping sp3 orbitals forming the C-C bond. The C-C bond is 1.54Å. As mentioned before, because of the free rotation about the C-C bond, ethane exists in many conformations (conformers)
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 02 Structure and Properties.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 01 Introduction.pdf
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 27 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 26 Orbitals and Organic Chemistry - Pericyclic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 25 Secondary Metabolites - An Introduction to Natural Products Chemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 24 Biomolecules - Nucleic Acids and Their Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 23 Biomolecules - Lipids and Their Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 22 Carbohydrate Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 21 Biomolecules - Carbohydrates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 20 Amino Acid Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 19 Biomolecules - Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 18 Amines and Heterocycles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 17 Carbonyl Alpha-Substitution and Condensation Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 16 Carboxylic Acid Derivatives - Nucleophilic Acyl Substitution Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 15 Carboxylic Acids and Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 14 Aldehydes and Ketones - Nucleophilic Additions Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 13 Alcohols, Phenols, and Thiols; Ethers and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 12 Organohalides - Nucleophilic Substitutions and Eliminations.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 10 Structure Determination - Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 11 Structure Determination - Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 04 Rates & Kinetics.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 05 Stereochemistry.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 06 Alkyl Halides.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 07 Structure and Synthesis of Alkenes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 08 Reactions of Alkenes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 09 Alkynes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 10 Synthesis and Structure of Alcohols.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 11 Reactions of Alcohols.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 13 Nuclear Magnetic Resonance(NMR)Spectroscopy.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 14 Ethers and Epoxides.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 15 Conjugated Systems.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 16 Aromatic Compounds.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 17 Reactions of Aromatic Compounds.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 18 Ketones and Aldehydes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 19 Amines.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 20 Carboxylic Acids.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 21 Carboxylic acid Derivatives.pdf
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)CHM 201 Introduction and Review - Structure and Bonding.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Structure and Bonding of Organic Molecules.pptx
