《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 15 Carboxylic Acids and Nitriles

CNGNGE JOHN MCMURRY CHAPTER 15 Carboxylic Acids and Nitriles EDITION Organic Chemistry with Biological Applications
CHAPTER 15 Carboxylic Acids and Nitriles
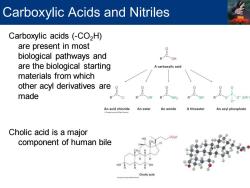
Carboxylic Acids and Nitriles Carboxylic acids (-CO2H) are present in most biological pathways and are the biological starting A carboxylic acid materials from which other acyl derivatives are made 。 0 O(OR' An acid chloride An ester An amide A thioester An acyl phosphate Cholic acid is a major component of human bile HO HO OH Cholic acid
Carboxylic acids (-CO2H) are present in most biological pathways and are the biological starting materials from which other acyl derivatives are made Cholic acid is a major component of human bile Carboxylic Acids and Nitriles
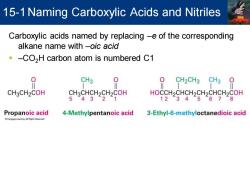
15-1 Naming Carboxylic Acids and Nitriles Carboxylic acids named by replacing -e of the corresponding alkane name with -oic acid -CO,H carbon atom is numbered C1 CH3 CH2CH3 CH3 CH3CH2COH CH3CHCH2CH2COH HOCCH2CHCH2CH2CHCH2COH 5432 12345678 Propanoic acid 4-Methylpentanoic acid 3-Ethyl-6-methyloctanedioic acid
Carboxylic acids named by replacing –e of the corresponding alkane name with –oic acid ▪ –CO2H carbon atom is numbered C1 15-1Naming Carboxylic Acids and Nitriles
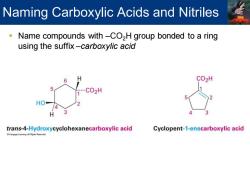
Naming Carboxylic Acids and Nitriles Name compounds with-CO2H group bonded to a ring using the suffix-carboxylic acid 6 CO2H HO trans-4-Hydroxycyclohexanecarboxylic acid Cyclopent-1-enecarboxylic acid
▪ Name compounds with –CO2H group bonded to a ring using the suffix –carboxylic acid Naming Carboxylic Acids and Nitriles
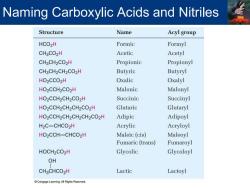
Naming Carboxylic Acids and Nitriles Structure Name Acyl group HCO2H Formic Formyl CH3CO2H Acetic Acetyl CH3CH2CO2H Propionic Propionyl CH3CH2CH2CO2H Butyric Butyryl HO2CCO2H Oxalic Oxalyl HO2CCH2CO2H Malonic Malonyl HO2CCH2CH2CO2H Succinic Succinyl HO2CCH2CH2CH2CO2H Glutaric Glutaryl HO2CCH2CH2CH2CH2CO2H Adipic Adipoyl H2C-CHCO2H Acrylic Acryloyl HO2CCH-CHCO2H Maleic(cis) Maleoyl Fumaric (trans) Fumaroyl HOCH2CO2H Glycolic Glycoloyl OH CH3CHCO2H Lactic Lactoyl Cengage Learning.All Rights Reserved
Naming Carboxylic Acids and Nitriles
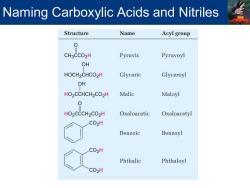
Naming Carboxylic Acids and Nitriles Structure Name Acyl group 0 CH3CCO2H Pyruvic Pyruvoyl OH HOCH2CHCO2H Glyceric Glyceroyl OH HO2CCHCH2CO2H Malic Maloyl 0 HO2CCCH2CO2H Oxaloacetic Oxaloacetyl CO2H Benzoic Benzoyl CO2H Phthalic Phthaloyl CO2H
Naming Carboxylic Acids and Nitriles

Naming Carboxylic Acids and Nitriles Nitriles,RCEN Compounds containing -C=N functional group are called nitriles Named by adding -nitrile as a suffix to the alkane name Nitrile carbon numbered C1 CH3 CH3CHCH2CH2CN 4-Methylpentanenitrile 54321
Nitriles, RC≡N Compounds containing -C≡N functional group are called nitriles ▪ Named by adding –nitrile as a suffix to the alkane name ▪ Nitrile carbon numbered C1 Naming Carboxylic Acids and Nitriles
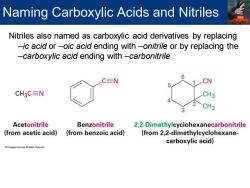
Naming Carboxylic Acids and Nitriles Nitriles also named as carboxylic acid derivatives by replacing -ic acid or-oic acid ending with-onitrile or by replacing the -carboxylic acid ending with-carbonitrile C三N CN CH3C=N CH3 Acetonitrile Benzonitrile 2,2-Dimethylcyclohexanecarbonitrile (from acetic acid) (from benzoic acid) (from 2,2-dimethylcyclohexane- carboxylic acid)
Nitriles also named as carboxylic acid derivatives by replacing –ic acid or –oic acid ending with –onitrile or by replacing the –carboxylic acid ending with –carbonitrile Naming Carboxylic Acids and Nitriles
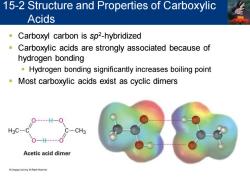
15-2 Structure and Properties of Carboxylic Acids Carboxyl carbon is sp2-hybridized a Carboxylic acids are strongly associated because of hydrogen bonding Hydrogen bonding significantly increases boiling point Most carboxylic acids exist as cyclic dimers O…H-O H3C-C C- CH3 Acetic acid dimer
▪ Carboxyl carbon is sp2 -hybridized ▪ Carboxylic acids are strongly associated because of hydrogen bonding ▪ Hydrogen bonding significantly increases boiling point ▪ Most carboxylic acids exist as cyclic dimers 15-2 Structure and Properties of Carboxylic Acids
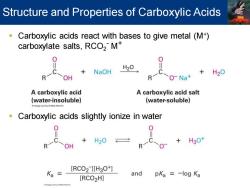
Structure and Properties of Carboxylic Acids Carboxylic acids react with bases to give metal(M+) carboxylate salts,RCO2M NaOH H20 +H20 OH R O-Na+ A carboxylic acid A carboxylic acid salt (water-insoluble) (water-soluble) Carboxylic acids slightly ionize in water R一 +H30 OH [RCO2 ][H3O+] Ka and pKa =-log Ka [RCO2H]
▪ Carboxylic acids react with bases to give metal (M+ ) carboxylate salts, RCO2 - M+ ▪ Carboxylic acids slightly ionize in water Structure and Properties of Carboxylic Acids
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 14 Aldehydes and Ketones - Nucleophilic Additions Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 13 Alcohols, Phenols, and Thiols; Ethers and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 12 Organohalides - Nucleophilic Substitutions and Eliminations.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 10 Structure Determination - Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 11 Structure Determination - Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 09 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 08 Reactions of Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 07 Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 06 An Overview of Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 05 Stereochemistry at Tetrahedral Centers.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 04 Organic Compounds - Cycloalkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 03 Organic Compounds - Alkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 02 Polar Covalent Bonds; Acids and Bases.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 01 Structure and Bonding.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Reaction Mechanism and Synthesis Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 16 Carboxylic Acid Derivatives - Nucleophilic Acyl Substitution Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 17 Carbonyl Alpha-Substitution and Condensation Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 18 Amines and Heterocycles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 19 Biomolecules - Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 20 Amino Acid Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 21 Biomolecules - Carbohydrates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 22 Carbohydrate Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 23 Biomolecules - Lipids and Their Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 24 Biomolecules - Nucleic Acids and Their Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 25 Secondary Metabolites - An Introduction to Natural Products Chemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 26 Orbitals and Organic Chemistry - Pericyclic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 27 Synthetic Polymers.ppt
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 01 Introduction.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 02 Structure and Properties.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 03 Alkanes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 04 Rates & Kinetics.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 05 Stereochemistry.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 06 Alkyl Halides.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 07 Structure and Synthesis of Alkenes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 08 Reactions of Alkenes.pdf
