《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 08 Reactions of Alkenes

Organic Chemistry, ORGANIC 8th Edition CHEMISTRY L.G.Wade,Jr. Chapter 8 Lecture Reactions of Alkenes G.WADE,JR Rizalia Klausmeyer Baylor University Waco,TX 2013 Pearson Education,Inc. ALWAYS LEARNING PEARSON
Chapter 8 Lecture Organic Chemistry, 8 th Edition L. G. Wade, Jr. Reactions of Alkenes © 2013 Pearson Education, Inc. Rizalia Klausmeyer Baylor University Waco, TX

Review of Substitution/Elimination Halide Type SN1 SN2 E1 E2 RCH2X (1) Does not occur Highly favored Does not occur Occurs when strong bases are used R2CHX (2) Can occur with Occurs in Can occur with Favored when strong benzylic and allylic competition with E2 benzylic and allylic bases are used halides halides R3CX(3】 Favored in hydroxylic Does not occur Occurs in Favored when bases solvents competition with S1 are used SN1 Reactions Works best when carbocation intermediate is stabilized by substitution Works best with strong leavinggroup(leaving group is stable in solvent) Frequently occur under neutral or acidic conditions Polar,protic solvents that stabilize nucleophile are best Works best with non-basic nucleophile SN2 Reactions Work faster in polar aprotic solvents(acetonitrile,acetone,DMF,DMSO) Work best with strong nucleophile that can reach carbon(e.g.,primary halide) Negatively-charged nucleophiles generally stronger than neutral nucleophiles Work best with good leaving group I<Br<Cl<F
Review of Substitution/Elimination 2 Halide Type SN1 SN2 E1 E2 RCH2X (1°) Does not occur Highly favored Does not occur Occurs when strong bases are used R2CHX (2°) Can occur with benzylic and allylic halides Occurs in competition with E2 Can occur with benzylic and allylic halides Favored when strong bases are used R3CX (3°) Favored in hydroxylic solvents Does not occur Occurs in competition with SN1 Favored when bases are used SN1 Reactions SN2 Reactions • Works best when carbocation intermediate is stabilized by substitution • Works best with strong leaving group (leaving group is stable in solvent) • Frequently occur under neutral or acidic conditions • Polar, protic solvents that stabilize nucleophile are best • Works best with non-basic nucleophile • Work faster in polar aprotic solvents (acetonitrile, acetone, DMF, DMSO) • Work best with strong nucleophile that can reach carbon (e.g., primary halide) • Negatively-charged nucleophiles generally stronger than neutral nucleophiles • Work best with good leaving group I < Br < Cl < F
Types of Alkene Reactions C=C The double-bond in alkenes make them chemically reactive (pi bond electrons held more loosely) Four major types of alkene reactions -Addition -Elimination -Substitution Rearrangement 3
Types of Alkene Reactions • The double-bond in alkenes make them chemically reactive (pi bond electrons held more loosely) • Four major types of alkene reactions – Addition – Elimination – Substitution – Rearrangement C=C 3
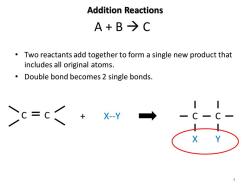
Addition Reactions A+B→C Two reactants add together to form a single new product that includes all original atoms. Double bond becomes 2 single bonds. Cc=c +Xy → X
Addition Reactions • Two reactants add together to form a single new product that includes all original atoms. • Double bond becomes 2 single bonds. A + B → C C C + X--Y C C X Y 4
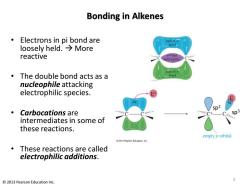
Bonding in Alkenes Electrons in pi bond are half of loosely held.→More bond reactive a bond held tightly half of开 The double bond acts as a bond nucleophile attacking electrophilic species. sp2 Carbocations are intermediates in some of these reactions. empty p orbital 2013Pearson Education,Inc. These reactions are called electrophilic additions. 5 2013 Pearson Education Inc
Bonding in Alkenes • Electrons in pi bond are loosely held. → More reactive • The double bond acts as a nucleophile attacking electrophilic species. • Carbocations are intermediates in some of these reactions. • These reactions are called electrophilic additions. © 2013 Pearson Education Inc. 5 sp2 sp3
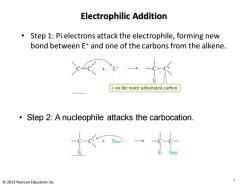
Electrophilic Addition Step 1:Pi electrons attack the electrophile,forming new bond between E+and one of the carbons from the alkene. on the more substituted carbon Step 2:A nucleophile attacks the carbocation. 6 2013 Pearson Education Inc
Electrophilic Addition • Step 1: Pi electrons attack the electrophile, forming new bond between E+ and one of the carbons from the alkene. • Step 2: A nucleophile attacks the carbocation. 6 © 2013 Pearson Education Inc

Types of Additions TABLE8-1 Types of Additions to Alkenes c=c Type of Addition [Elements Added]a Product OH hydration halogenation [H,O] IX.I,an oxidation hydrogenation halohydrin formation [H].a reduction [HOX],an oxidation 平 dihydroxylation HX addition [HOOH],an oxidation HX灯 4 (hydrohalogenation) oxidative cleavage C=0 [O].an oxidation 0=0 cyclopropanation epoxidation [CH] [O].an oxidation "These are not the reagents used but simply the groups that appear in the product. 0口ertm Educatm Ie 7 2013 Pearson Education Inc
Types of Additions 7 © 2013 Pearson Education Inc

Mechanism of Addition of HX(HBr,HCl,HI) Step 1:Protonation of the double bond (Nu-attacks H+) HH HH CH3一C〒C-CH CH3-C-C-CH3+:Br: H Stability of carbocations:.3°>2°>1。>+CH3 An electrophile adds to a double bond to give most stable intermediate carbocation. Step 2:Nucleophilic attack of the halide on the carbocation. HH HH CH3一C-C一CH3+:Br CH3-C-C- CH H H :Br: 2013 Pearson Education Inc
Mechanism of Addition of HX (HBr, HCl, HI) Step 1: Protonation of the double bond (Nu- attacks H+). Step 2: Nucleophilic attack of the halide on the carbocation. 8 © 2013 Pearson Education Inc. Stability of carbocations: 3° > 2° > 1° > +CH3 An electrophile adds to a double bond to give most stable intermediate carbocation

Selective Addition:Markovnikov's Rule 。 Markovnikov's rule:Proton added to double bond of an alkene goes to the carbon atom that already holds the greater number of hydrogens. Markovnikov's rule (extended):In an electrophilic addition to the alkene,the electrophile adds in such a way that it generates the most stable intermediate. CHs CHs CH3-C-CH-CH3 add Hto secondary carbon CH,-C-CH-CH, →HBr H Br tertiary carbocation CH3 CH一CCH-CH add H to tertiary carbon CH,-C-CH-CH; H Br secondary carbocation 9 2013 Pearson Education Inc
Selective Addition: Markovnikov’s Rule • Markovnikov’s rule: Proton added to double bond of an alkene goes to the carbon atom that already holds the greater number of hydrogens. • Markovnikov’s rule (extended): In an electrophilic addition to the alkene, the electrophile adds in such a way that it generates the most stable intermediate. 9 © 2013 Pearson Education Inc
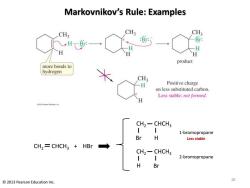
Markovnikov's Rule:Examples CH3 CH3 CH3 : H H product more bonds to hydrogen CH3 H Positive charge on less substituted carbon. Less stable:not formed. CH2-CHCH3 1-bromopropane Br H Less stable CH,=CHCH3 HBr CH2-CHCH3 2-bromopropane H Br 10 2013 Pearson Education Inc
Markovnikov’s Rule: Examples 10 © 2013 Pearson Education Inc. CH2 CHCH3 Br + H HBr CH2 CHCH3 H Br CH2 CHCH3 1-bromopropane 2-bromopropane Less stable
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 22 Condensations and Alpha Substitutions of Carbonyl Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 21 Part 1 - Structure and Properties of Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 13 Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Reaction Mechanism and Synthesis Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 01 Structure and Bonding.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 02 Polar Covalent Bonds; Acids and Bases.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 03 Organic Compounds - Alkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 04 Organic Compounds - Cycloalkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 05 Stereochemistry at Tetrahedral Centers.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 06 An Overview of Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 07 Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 08 Reactions of Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 09 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 11 Structure Determination - Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 10 Structure Determination - Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 12 Organohalides - Nucleophilic Substitutions and Eliminations.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 13 Alcohols, Phenols, and Thiols; Ethers and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 14 Aldehydes and Ketones - Nucleophilic Additions Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 15 Carboxylic Acids and Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 16 Carboxylic Acid Derivatives - Nucleophilic Acyl Substitution Reactions.ppt
