《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 07 Alkenes and Alkynes

CNGNGE JOHN MCMURRY CHAPTER 7 Alkenes and Alkynes EDITION Organic Chemistry with Biological Applications
CHAPTER 7 Alkenes and Alkynes
Alkenes and Alkynes Alkene (or olefin) Hydrocarbon that contains a carbon-carbon double bond Present in most organic and biological molecules Alkyne Hydrocarbon that contains a carbon-carbon triple bond Rarely occur in biological molecules or pathways
Alkenes and Alkynes Alkene (or olefin) ▪ Hydrocarbon that contains a carbon-carbon double bond ▪ Present in most organic and biological molecules Alkyne ▪ Hydrocarbon that contains a carbon-carbon triple bond ▪ Rarely occur in biological molecules or pathways
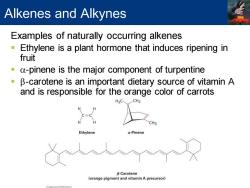
Alkenes and Alkynes Examples of naturally occurring alkenes Ethylene is a plant hormone that induces ripening in fruit a-pinene is the major component of turpentine B-carotene is an important dietary source of vitamin A and is responsible for the orange color of carrots CH3 Ethylene a-Pinene B-Carotene (orange pigment and vitamin A precursor)
Alkenes and Alkynes Examples of naturally occurring alkenes ▪ Ethylene is a plant hormone that induces ripening in fruit ▪ a-pinene is the major component of turpentine ▪ b-carotene is an important dietary source of vitamin A and is responsible for the orange color of carrots
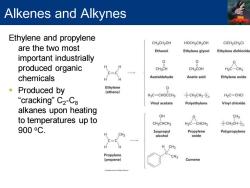
Alkenes and Alkynes Ethylene and propylene CH2CH2OH HOCH2CH2OH CICH2CH2CI are the two most Ethanol Ethylene glycol Ethylene dichloride important industrially 0 0 produced organic CHaCH CH3COH H2C-CH2 c=c chemicals H Acetaldehyde Acetic acid Ethylene oxide Produced by Ethylene 0 (ethene】 H2C=CHOCCH3 之CH2CH2之n H2C=CHCI “cracking”C2-C8 Vinyl acetate Polyethylene Vinyl chloride alkanes upon heating OH 0 CH3 to temperatures up to CH3CHCH3 H2C-CHCH3 之CH2CHzn 900oC. Isopropyl Propylene Polypropylene CH3 alcohol oxide H CH3 Propylene CH3 (propene) Cumene
Alkenes and Alkynes Ethylene and propylene are the two most important industrially produced organic chemicals ▪ Produced by “cracking” C2 -C8 alkanes upon heating to temperatures up to 900 oC

7-1 Calculating a Degree of Unsaturation Because of its double bond an alkene has fewer hydrogens than an alkane with the same number of carbons and is therefore referred to as unsaturated Alkene Alkane CnH2n CnH2n+2 C2H4 C2H6 H Ethylene:C2H4 Ethane:C2H6 (Fewer hydrogens-Unsaturated) (More hydrogens-Saturated)
Because of its double bond an alkene has fewer hydrogens than an alkane with the same number of carbons and is therefore referred to as unsaturated Alkene Alkane CnH2n CnH2n+2 C2H4 C2H6 7-1 Calculating a Degree of Unsaturation
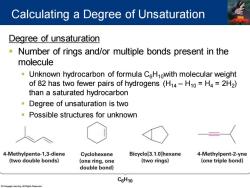
Calculating a Degree of Unsaturation Degree of unsaturation Number of rings and/or multiple bonds present in the molecule Unknown hydrocarbon of formula CeHnowith molecular weight of 82 has two fewer pairs of hydrogens(H14-H10=H4=2H2) than a saturated hydrocarbon Degree of unsaturation is two Possible structures for unknown 4-Methylpenta-1,3-diene Cyclohexene Bicyclo[3.1.0]hexane 4-Methylpent-2-yne (two double bonds) (one ring,one (two rings) (one triple bond) double bond) C6H10
Degree of unsaturation ▪ Number of rings and/or multiple bonds present in the molecule ▪ Unknown hydrocarbon of formula C6H10with molecular weight of 82 has two fewer pairs of hydrogens (H14 – H10 = H4 = 2H2 ) than a saturated hydrocarbon ▪ Degree of unsaturation is two ▪ Possible structures for unknown Calculating a Degree of Unsaturation
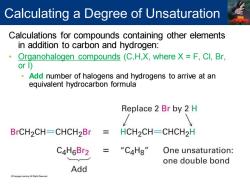
Calculating a Degree of Unsaturation Calculations for compounds containing other elements in addition to carbon and hydrogen: Organohalogen compounds (C,H,X,where X F,Cl,Br, or 1) Add number of halogens and hydrogens to arrive at an equivalent hydrocarbon formula Replace 2 Br by 2 H BrCH2CH-CHCH2Br HCH2CH-CHCH2H C4H6Br2 “C4H8" One unsaturation: one double bond Add LeamingAl Pigh Reer时
Calculations for compounds containing other elements in addition to carbon and hydrogen: • Organohalogen compounds (C,H,X, where X = F, Cl, Br, or I) • Add number of halogens and hydrogens to arrive at an equivalent hydrocarbon formula Calculating a Degree of Unsaturation
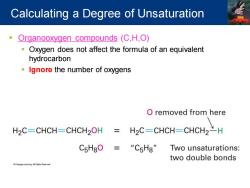
Calculating a Degree of Unsaturation Organooxygen compounds(C,H,O) Oxygen does not affect the formula of an equivalent hydrocarbon Ignore the number of oxygens O removed from here H2C=CHCH-CHCH2OH 三 H2C=CHCH-CHCH2H C5HgO=“CsHg”Two unsaturations:: two double bonds
▪ Organooxygen compounds (C,H,O) ▪ Oxygen does not affect the formula of an equivalent hydrocarbon ▪ Ignore the number of oxygens Calculating a Degree of Unsaturation
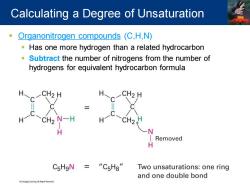
Calculating a Degree of Unsaturation Organonitrogen compounds (C,H,N) Has one more hydrogen than a related hydrocarbon Subtract the number of nitrogens from the number of hydrogens for equivalent hydrocarbon formula H CH2 H H CH2 H CH2 N-H H CH2 H N Removed H C5HgN=“CsHg" Two unsaturations:one ring and one double bond Cngg Learing Al Rig Raserved
▪ Organonitrogen compounds (C,H,N) ▪ Has one more hydrogen than a related hydrocarbon ▪ Subtract the number of nitrogens from the number of hydrogens for equivalent hydrocarbon formula Calculating a Degree of Unsaturation
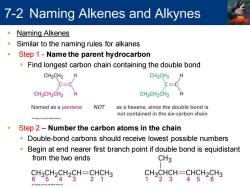
7-2 Naming Alkenes and Alkynes Naming Alkenes Similar to the naming rules for alkanes Step 1-Name the parent hydrocarbon Find longest carbon chain containing the double bond CH3CH2 H CH3CH2 C=C C=C CH3CH2CH2 H CH3CH2CH2 H Named as a pentene NOT as a hexene,since the double bond is not contained in the six-carbon chain OCgLai eat Step 2-Number the carbon atoms in the chain Double-bond carbons should receive lowest possible numbers Begin at end nearer first branch point if double bond is equidistant from the two ends CH3 CH3CH2CH2CH=CHCH3 CH3CHCH=CHCH2CH3 654321 123456
7-2 Naming Alkenes and Alkynes ▪ Naming Alkenes ▪ Similar to the naming rules for alkanes ▪ Step 1 - Name the parent hydrocarbon ▪ Find longest carbon chain containing the double bond ▪ Step 2 – Number the carbon atoms in the chain ▪ Double-bond carbons should receive lowest possible numbers ▪ Begin at end nearer first branch point if double bond is equidistant from the two ends
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 06 An Overview of Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 05 Stereochemistry at Tetrahedral Centers.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 04 Organic Compounds - Cycloalkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 03 Organic Compounds - Alkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 02 Polar Covalent Bonds; Acids and Bases.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 01 Structure and Bonding.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Reaction Mechanism and Synthesis Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 08 Reactions of Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 09 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 11 Structure Determination - Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 10 Structure Determination - Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 12 Organohalides - Nucleophilic Substitutions and Eliminations.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 13 Alcohols, Phenols, and Thiols; Ethers and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 14 Aldehydes and Ketones - Nucleophilic Additions Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 15 Carboxylic Acids and Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 16 Carboxylic Acid Derivatives - Nucleophilic Acyl Substitution Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 17 Carbonyl Alpha-Substitution and Condensation Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 18 Amines and Heterocycles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 19 Biomolecules - Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 20 Amino Acid Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 21 Biomolecules - Carbohydrates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 22 Carbohydrate Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 23 Biomolecules - Lipids and Their Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 24 Biomolecules - Nucleic Acids and Their Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 25 Secondary Metabolites - An Introduction to Natural Products Chemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 26 Orbitals and Organic Chemistry - Pericyclic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 27 Synthetic Polymers.ppt
