《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 22 Carbohydrate Metabolism

CNGNGE JOHN MCMURRY CHAPTER 22 Carbohydrate Metabolism T H IR D E DI TION Organic Chemistry with Biological applications
CHAPTER 22 Carbohydrate Metabolism
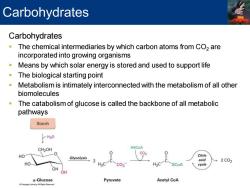
Carbohydrates Carbohydrates The chemical intermediaries by which carbon atoms from CO2 are incorporated into growing organisms Means by which solar energy is stored and used to support life The biological starting point Metabolism is intimately interconnected with the metabolism of all other biomolecules The catabolism of glucose is called the backbone of all metabolic pathways Starch H20 CH2OH HSCoA c02 HO Citric Glycolysis 2 acid →2c02 HO- H3C SCoA cycle OH OH a-Glucose Pyruvate Acetyl CoA LeamingAll Pighis Peservd
Carbohydrates ▪ The chemical intermediaries by which carbon atoms from CO2 are incorporated into growing organisms ▪ Means by which solar energy is stored and used to support life ▪ The biological starting point ▪ Metabolism is intimately interconnected with the metabolism of all other biomolecules ▪ The catabolism of glucose is called the backbone of all metabolic pathways Carbohydrates

22-1 Hydrolysis of Complex Carbohydrates Carbohydrate found in food is mostly starch A glucose polymer Monosaccharide units are linked by a-(1-4)glycoside bonds Consists of two main fractions 1.Amylose Makes up 20%of starch by mass A linear polymer of several hundred a-(1-4)-linked glucose units 2.Amylopectin Makes up 80%of starch by mass A branched polymer of up to 5000 glucose units with a- (1-6)branches about every 25 units
Carbohydrate found in food is mostly starch ▪ A glucose polymer ▪ Monosaccharide units are linked by a-(1→4) glycoside bonds ▪ Consists of two main fractions 1. Amylose ▪ Makes up 20% of starch by mass ▪ A linear polymer of several hundred a-(1→4)-linked glucose units 2. Amylopectin ▪ Makes up 80% of starch by mass ▪ A branched polymer of up to 5000 glucose units with a- (1→6) branches about every 25 units 22-1Hydrolysis of Complex Carbohydrates
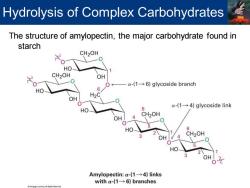
Hydrolysis of Complex Carbohydrates The structure of amylopectin,the major carbohydrate found in starch CH2OH HO- CH2OH OH 0← a-(1-6)glycoside branch HO 6 H2C OH a-(1-4)glycoside link HO 6 CH2OH 6 CH2OH HO Amylopectin:a-(1-4)links with a-(1-6)branches
The structure of amylopectin, the major carbohydrate found in starch Hydrolysis of Complex Carbohydrates
Hydrolysis of Complex Carbohydrates Digestion of starches Begins in the mouth Many of the internal(1-4)glycoside links are randomly hydrolyzed by a-amylase,a glycosidase Continues in the small intestines Gives a mixture of disaccharide maltose,trisaccharide maltotriose,and small oligosaccharides called limit dextrins, which contain (1-6)branches Final processing in the intestinal mucosa by additional glycosidases Yields glucose which is absorbed by the intestine and transported through the bloodstream
Digestion of starches ▪ Begins in the mouth ▪ Many of the internal (1→4) glycoside links are randomly hydrolyzed by a-amylase, a glycosidase ▪ Continues in the small intestines ▪ Gives a mixture of disaccharide maltose, trisaccharide maltotriose, and small oligosaccharides called limit dextrins, which contain (1→6) branches ▪ Final processing in the intestinal mucosa by additional glycosidases ▪ Yields glucose which is absorbed by the intestine and transported through the bloodstream Hydrolysis of Complex Carbohydrates
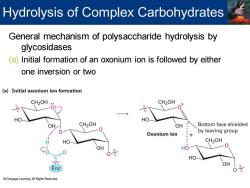
Hydrolysis of Complex Carbohydrates General mechanism of polysaccharide hydrolysis by glycosidases (a)Initial formation of an oxonium ion is followed by either one inversion or two (a)Initial oxonium ion formation CH2OH CH2OH OH CH2OH OH Bottom face shielded Oxonium ion by leaving group HO CH2OH OH HO 0之 HO Enz Cengage Learning.All Rights Reserved
General mechanism of polysaccharide hydrolysis by glycosidases (a) Initial formation of an oxonium ion is followed by either one inversion or two Hydrolysis of Complex Carbohydrates
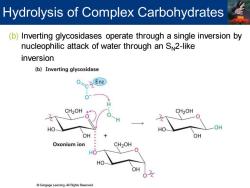
Hydrolysis of Complex Carbohydrates (b)Inverting glycosidases operate through a single inversion by nucleophilic attack of water through an SN2-like inversion (b)Inverting glycosidase Enz CH2OH CH2OH HO HO OH OH OH Oxonium ion CH2OH HO -0 HO Cengage Learning.All Rights Reserved
(b) Inverting glycosidases operate through a single inversion by nucleophilic attack of water through an SN2-like inversion Hydrolysis of Complex Carbohydrates
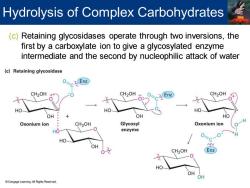
Hydrolysis of Complex Carbohydrates (c)Retaining glycosidases operate through two inversions,the first by a carboxylate ion to give a glycosylated enzyme intermediate and the second by nucleophilic attack of water (c)Retaining glycosidase CZ Enz CH2OH CH2OH Enz CH2OH HO HO HO OH OH HO Oxonium ion CH2OH Glycosyl Oxonium ion HO enzyme 0 HO OH 0 CH2OH Enz HO OH OH Cengage Learning.All Rights Reserved
(c) Retaining glycosidases operate through two inversions, the first by a carboxylate ion to give a glycosylated enzyme intermediate and the second by nucleophilic attack of water Hydrolysis of Complex Carbohydrates

22-2 Catabolism of Glucose:Glycolysis Glycolysis Beginning of catabolsim for glucose -A series of ten enzyme-catalyzed reactions that break down glucose into 2 equivalents of pyruvate, CH.COCO2 Steps are called the Embden-Meyerhoff pathway
Glycolysis ▪ Beginning of catabolsim for glucose ▪ A series of ten enzyme-catalyzed reactions that break down glucose into 2 equivalents of pyruvate, CH3COCO2 - ▪ Steps are called the Embden-Meyerhoff pathway 22-2 Catabolism of Glucose: Glycolysis

Catabolism of Glucose:Glycolysis CH2OH Figure 22.3 HO a-Glucose The 10-step Glucose is phosphorylated by reaction with ATP to yield glucose 6-phosphate. glycolysis hohte HO pathway for OH -0H catabolizing Glucose 6-phosphate is isomerized to fructose 6-phosphate by ring opening followed by a keto-enol tautomerization. glucose to two CH.OH C=0 molecules of OH -OH pyruvate Fructose 6-phosphate is phosphorylated by reaction with ATP toyield fructose 1.6-bisphosphate C=0 HO- -H H -OH Fructose 1,6-bisphosphate undergoes ring OH opening and is cleaved by a retro-aldol reaction into glyceraldehyde 3-phosphate and dihydroxyacetone phosphate(DHAP) 3-phosphate. CH.OH H00 c=0 O POCH CHCH 5 -OH
Figure 22.3 The 10-step glycolysis pathway for catabolizing glucose to two molecules of pyruvate Catabolism of Glucose: Glycolysis
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 21 Biomolecules - Carbohydrates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 20 Amino Acid Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 19 Biomolecules - Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 18 Amines and Heterocycles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 17 Carbonyl Alpha-Substitution and Condensation Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 16 Carboxylic Acid Derivatives - Nucleophilic Acyl Substitution Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 15 Carboxylic Acids and Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 14 Aldehydes and Ketones - Nucleophilic Additions Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 13 Alcohols, Phenols, and Thiols; Ethers and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 12 Organohalides - Nucleophilic Substitutions and Eliminations.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 10 Structure Determination - Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 11 Structure Determination - Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 09 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 08 Reactions of Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 07 Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 06 An Overview of Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 05 Stereochemistry at Tetrahedral Centers.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 04 Organic Compounds - Cycloalkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 03 Organic Compounds - Alkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 02 Polar Covalent Bonds; Acids and Bases.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 23 Biomolecules - Lipids and Their Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 24 Biomolecules - Nucleic Acids and Their Metabolism.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 25 Secondary Metabolites - An Introduction to Natural Products Chemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 26 Orbitals and Organic Chemistry - Pericyclic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 27 Synthetic Polymers.ppt
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 01 Introduction.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 02 Structure and Properties.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 03 Alkanes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 04 Rates & Kinetics.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 05 Stereochemistry.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 06 Alkyl Halides.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 07 Structure and Synthesis of Alkenes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 08 Reactions of Alkenes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 09 Alkynes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 10 Synthesis and Structure of Alcohols.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 11 Reactions of Alcohols.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 13 Nuclear Magnetic Resonance(NMR)Spectroscopy.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 14 Ethers and Epoxides.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 15 Conjugated Systems.pdf
