《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy

Organic Chemistry, ORGANIC 8th Edition CHEMISTRY L.G.Wade,Jr. Chapter 15 Lecture Conjugated Systems, Orbital Symmetry,and Ultraviolet Spectroscopy G.WADE,J R 2013 Pearson Education,Inc. ALWAYS LEARNING PEARSON
© 2013 Pearson Education, Inc. Chapter 15 1 Chapter 15 Lecture Organic Chemistry, 8 th Edition L. G. Wade, Jr. Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy © 2013 Pearson Education, Inc

Conjugated Systems c-c-☒ conjugated double bonds penta-1,3-diene (more stable than isolated double bonds) H、 H、 H >c=Cc=c< CH, CH2 isolated double bonds penta-1,4-diene Conjugated double bonds are separated by one single bond. Isolated double bonds are separated by two or more single bonds Conjugated double bonds are more stable than isolated ones. 2013 Pearson Education,Inc. Chapter 15
© 2013 Pearson Education, Inc. Chapter 15 2 Conjugated Systems • Conjugated double bonds are separated by one single bond. • Isolated double bonds are separated by two or more single bonds. • Conjugated double bonds are more stable than isolated ones
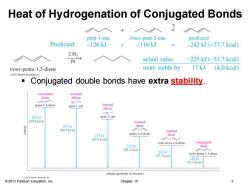
Heat of Hydrogenation of Conjugated Bonds ◇入 pent-1-ene trans-pent-2-ene predicted Predicted: -126kJ -116kJ -242kJ(-57.7kcal) 2H2 Pt actual value -225kJ(-53.7kcal) trans-penta-1,3-diene more stable by 17 kJ (4.0 kcal) Conjugated double bonds have extra stability. cumulated terminal diene alkyne penta-1.2-diene pent-1-yne alkyne 202kJ pent-2-yne (69.8 keal isolated 到 291kJ diene (69.5 keal) isolated 275k penta-1.4-dien diene (65.8 keal) 入 trans-hexa-1,4-dicne conjugated 252」 diene (60.2 keal 242 (57.7 keal trans-penta-1.3-diene 225J (53.7 kcal)- alkane (pentane or hexane) 2013 Pearson Education,Inc. Chapter 15 3
© 2013 Pearson Education, Inc. Chapter 15 3 Heat of Hydrogenation of Conjugated Bonds ▪ Conjugated double bonds have extra stability
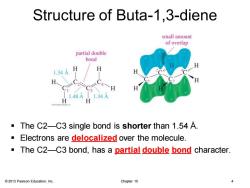
Structure of Buta-1.3-diene small amount of overlap partial double bond H 1.34A 1.48A L.34A The C2-C3 single bond is shorter than 1.54 A. Electrons are delocalized over the molecule. The C2-C3 bond,has a partial double bond character. 2013 Pearson Education,Inc. Chapter 15
© 2013 Pearson Education, Inc. Chapter 15 4 Structure of Buta-1,3-diene ▪ The C2—C3 single bond is shorter than 1.54 Å. ▪ Electrons are delocalized over the molecule. ▪ The C2—C3 bond, has a partial double bond character
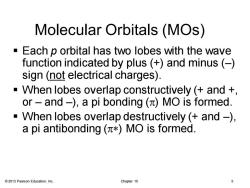
Molecular Orbitals(MOs) -Each p orbital has two lobes with the wave function indicated by plus (+)and minus(-) sign (not electrical charges). -When lobes overlap constructively (and + or-and-),a pi bonding (MO is formed. -When lobes overlap destructively (and-), a pi antibonding(π*)MO is formed. 2013 Pearson Education,Inc. Chapter 15 5
© 2013 Pearson Education, Inc. Chapter 15 5 Molecular Orbitals (MOs) ▪ Each p orbital has two lobes with the wave function indicated by plus (+) and minus (–) sign (not electrical charges). ▪ When lobes overlap constructively (+ and +, or – and –), a pi bonding (p) MO is formed. ▪ When lobes overlap destructively (+ and –), a pi antibonding (p*) MO is formed
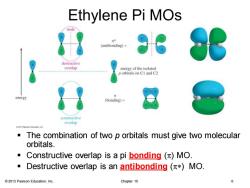
Ethylene Pi MOs node (antibonding)= destructive overlap energy of the isolated orbitals on CI and C2 energy (bonding)= constructive overlap The combination of two p orbitals must give two molecular orbitals. ■ Constructive overlap is a pi bonding()MO. Destructive overlap is an antibonding (*MO. 2013 Pearson Education,Inc. Chapter 15
© 2013 Pearson Education, Inc. Chapter 15 6 Ethylene Pi MOs ▪ The combination of two p orbitals must give two molecular orbitals. ▪ Constructive overlap is a pi bonding (p) MO. ▪ Destructive overlap is an antibonding (p*) MO
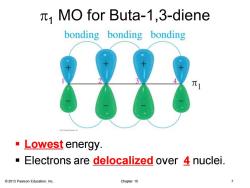
MO for Buta-1,3-diene bonding bonding bonding ·Lowest energy. Electrons are delocalized over 4 nuclei. 2013 Pearson Education,Inc. Chapter 15
© 2013 Pearson Education, Inc. Chapter 15 7 p1 MO for Buta-1,3-diene ▪ Lowest energy. ▪ Electrons are delocalized over 4 nuclei
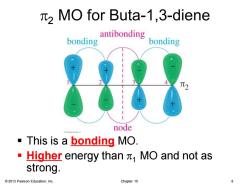
2 MO for Buta-1,3-diene bonding antibonding bonding node This is a bonding MO. Higher energy than MO and not as strong. 2013 Pearson Education,Inc. Chapter 15
© 2013 Pearson Education, Inc. Chapter 15 8 p2 MO for Buta-1,3-diene ▪ This is a bonding MO. ▪ Higher energy than p1 MO and not as strong
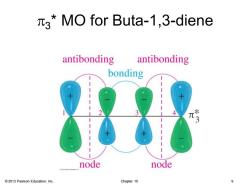
3*MO for Buta-1,3-diene antibonding antibonding bonding 3 node node 2013 Pearson Education,Inc. Chapter 15 9
© 2013 Pearson Education, Inc. Chapter 15 9 p3 * MO for Buta-1,3-diene
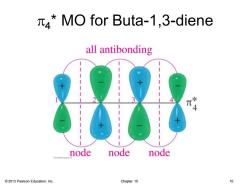
MO for Buta-1,3-diene all antibonding 4 node node node 2013 Pearson Education,Inc. Chapter 15 10
© 2013 Pearson Education, Inc. Chapter 15 10 p4 * MO for Buta-1,3-diene
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 08 Reactions of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 07 Structure and Synthesis of Alkenes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 06 Alkyl Halides - Nucleophilic Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 05 Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 03 Structure and Stereochemistry of Alkanes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 02 Structure and Properties of Organic Molecules.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 01 Introduction and Review.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 26 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 25 Lipids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 24 Amino Acids, Peptides, and Proteins.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 23 Carbohydrates and Nucleic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 22 Condensations and Alpha Substitutions of Carbonyl Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 21 Part 1 - Structure and Properties of Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 20 Carboxylic Acids.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 19 Amines.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 18 Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 17 Reactions of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 7th Edition, L. G. Wade, Jr.Pearson Education)Chapter 14 Ethers, Epoxides, and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 16 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Chapter 04 The Study of Chemical Reactions.ppt
- 《有机化学》课程PPT教学课件(Official PPT of Organic Chemistry, 8th Edition, L. G. Wade, Jr.Pearson Education)Reaction Mechanism and Synthesis Review.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 01 Structure and Bonding.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 02 Polar Covalent Bonds; Acids and Bases.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 03 Organic Compounds - Alkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 04 Organic Compounds - Cycloalkanes and Their Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 05 Stereochemistry at Tetrahedral Centers.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 06 An Overview of Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 07 Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 08 Reactions of Alkenes and Alkynes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 09 Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 11 Structure Determination - Nuclear Magnetic Resonance Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 10 Structure Determination - Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 12 Organohalides - Nucleophilic Substitutions and Eliminations.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 13 Alcohols, Phenols, and Thiols; Ethers and Sulfides.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 14 Aldehydes and Ketones - Nucleophilic Additions Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 15 Carboxylic Acids and Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 16 Carboxylic Acid Derivatives - Nucleophilic Acyl Substitution Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 17 Carbonyl Alpha-Substitution and Condensation Reactions.ppt
